- Title
-
Functional Characterization of Clinical Isolates of the Opportunistic Fungal Pathogen Aspergillus nidulans
- Authors
- Bastos, R.W., Valero, C., Silva, L.P., Schoen, T., Drott, M., Brauer, V., Silva-Rocha, R., Lind, A., Steenwyk, J.L., Rokas, A., Rodrigues, F., Resendiz-Sharpe, A., Lagrou, K., Marcet-Houben, M., Gabaldón, T., McDonnell, E., Reid, I., Tsang, A., Oakley, B.R., Loures, F.V., Almeida, F., Huttenlocher, A., Keller, N.P., Ries, L.N.A., Goldman, G.H.
- Source
- Full text @ mSphere
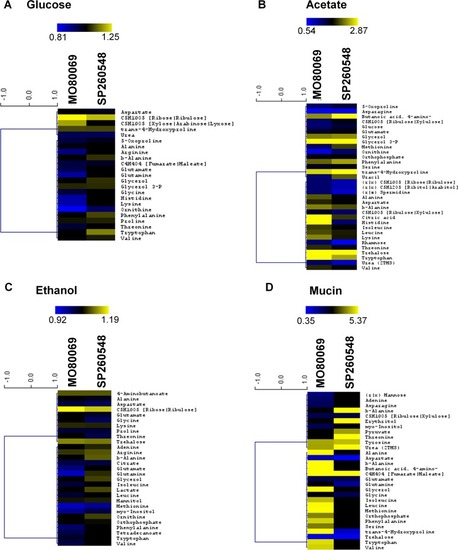
|
The |
|
The |
|
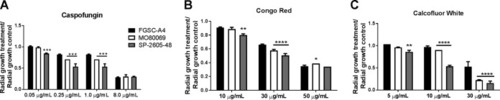
The A. nidulans clinical isolates are more sensitive to the cell wall-perturbing agents. (A to C) Strains were grown from 105 spores on glucose minimal medium supplemented with increasing concentrations of caspofungin, Congo red, and calcofluor white for 5 days at 37°C. Standard deviations represent biological triplicates in a two-way ANOVA test, comparing growth of the clinical isolates to growth of the FGSC A4 reference strain (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001). |
|
Diagram depicting the location of all detected nonsynonymous single nucleotide polymorphisms (SNPs) on the 8 chromosomes (Chr I to Chr VIII) of the |
|
Diagram depicting the location of all detected small deletions on the 8 chromosomes (Chr I to Chr VIII) of the |
|
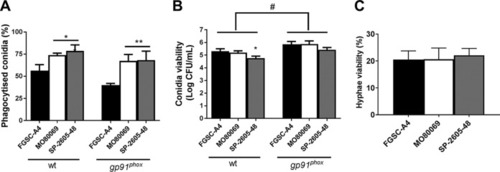
MpkA is not phosphorylated in the |
|
The |
|
A. nidulans strain-specific virulence depends on the host immune status. The virulence of the A. nidulans clinical isolates MO80069 and SP-260548 was tested in murine (A, C, and E) and zebrafish (B, D, and F) models of pulmonary and invasive aspergillosis. Animals were manipulated in order to give rise to either immunocompetent (A and B), CGD (chronic granulomatous disease) (C and D), or neutropenic (E)/neutrophilic (F) models. Shown are survival curves for each immunosuppression condition and animal model. No difference in virulence levels was detected for all strains in both immunocompetent and CGD mice. Strain MO80069 was significantly more virulent in neutropenic mice and neutrophilic zebrafish. **, P < 0.01; ****, P < 0.0001 for a comparison of the values for the clinical isolates to those of the FGSC A4 reference strain in a two-way ANOVA test with Tukey’s posttest. PHENOTYPE:
|