- Title
-
Pumilio response and AU-rich elements drive rapid decay of Pnrc2-regulated cyclic gene transcripts
- Authors
- Tietz, K.T., Gallagher, T.L., Mannings, M.C., Morrow, Z.T., Derr, N.L., Amacher, S.L.
- Source
- Full text @ Dev. Biol.
|
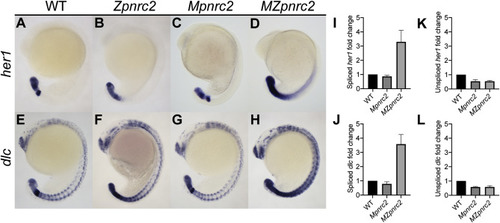
Fig. 1. Maternal and zygotic pnrc2 promotes proper her1 and dlc expression. Wild-type (WT), zygotic pnrc2oz22 (Zpnrc2), maternal pnrc2oz22 (Mpnrc2), and maternal-zygotic pnrc2oz22 (MZpnrc2) mutant embryos were raised to mid-segmentation stage (16–18 hpf) and probed for her1 (A–D) and dlc expression (E–F) by in situ hybridization (n ≥ 7 each). WT, Mpnrc2 mutant, and MZpnrc2 mutant embryos (n = 10 per biological replicate) were analyzed by qPCR using primers to amplify across exon-exon boundaries to detect spliced her1 (I) and dlc (J) transcripts or primers to amplify across intron-exon boundaries to detect her1 (K) and dlc (L) unspliced transcripts. MZpnrc2 mutant embryos have ~4-fold higher levels of spliced her1 and dlc mRNA than wild-type or Mpnrc2 mutant embryos, which have comparable levels (I and J). Both MZpnrc2 and Mpnrc2 mutant embryos have ~2-fold less unspliced her1 and dlc transcripts compared to WT embryos (K and L). hpf = hours post-fertilization. |
|
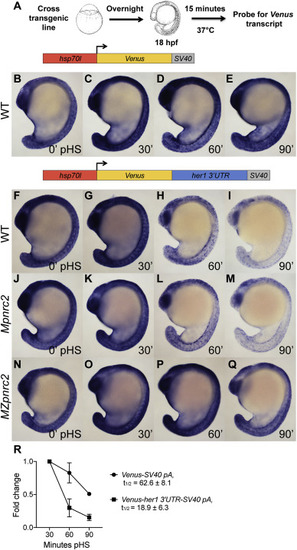
Fig. 2. The her1 3′UTR confers Pnrc2-mediated instability to reporter transcripts. (A) Diagram illustrating the heat shock protocol used for transgenic lines in this study. (B–I) Transgenic embryos carrying the hsp70l:Venus-her1 3′UTR-SV40 pA reporter (line oz44) or hsp70l:Venus-SV40 pA reporter (line oz68) were raised to mid-segmentation stage, heat shocked for 15 min, then collected at the indicated minutes pHS and processed by Venus in situ hybridization (n ≥ 7 embryos per time point). Venus transcript is not detected in the absence of heat shock (n = 10 per reporter line) (data not shown). (J–Q) Mid-segmentation stage Mpnrc2 mutant embryos and MZpnrc2 mutant embryos carrying the hsp70l:Venus-her1 3′UTR-SV40 pA reporter (line oz44) were heat shocked and processed by Venus in situ hybridization as above (n ≥ 8 embryos per time point). Representative embryos were genotyped post-imaging to confirm genotype. (R) qPCR analysis comparing Venus transcript fold change from 30 min pHS to 60 and 90 min pHS for the Tg(hsp70l:Venus-her1 3′UTR-SV40 pA)oz44 and Tg(hsp70l:Venus-SV40 pA)oz68 reporter lines (n = 10 embryos per time point across three biological replicates). Three independent lines carrying the hsp70l:Venus-her1 3′UTR-SV40 pA reporter and five independent lines carrying the hsp70l:Venus-SV40 pA reporter were analyzed in wild-type embryos by in situ hybridization and exhibited comparable Venus decay across all lines carrying the same reporter (data not shown); one representative line for each is shown (see Methods for details). For the hsp70l:Venus-her1 3′UTR-SV40 pA reporter, three independent lines were analyzed by qPCR and exhibited comparable decay (Supplemental Fig. 2). pHS = post-heat shock; hpf = hours post-fertilization; t1/2 = half-life; ± = standard deviation; pA = polyadenylation sequence. |
|
Fig. 3. The terminal 179 nucleotides of the her1 3′UTR is sufficient for Pnrc2-mediated decay of reporter transcripts. (A–F) Transgenic embryos carrying the hsp70l:Venus-her1 3′UTRΔ363-725-SV40 pA reporter (line oz54) or hsp70l:Venus-her1 3′UTRΔ1-362-SV40 pA reporter (line oz47) were raised to mid-segmentation stage and heat shocked for 15 min, then collected at the indicated minutes pHS and processed by Venus in situ hybridization (n ≥ 6 per time point). Venus transcript is not detected in the absence of heat shock (n = 10 per reporter line) (data not shown). (G) qPCR analysis comparing Venus transcript fold change from 30 min pHS to 60 and 90 min pHS for the Tg(hsp70l:Venus-her1 3′UTRΔ363-725-SV40 pA)oz54 or Tg(hsp70l:Venus-her1 3′UTRΔ1-362-SV40 pA)oz47 reporter lines (n = 10 embryos per time point across three biological replicates). (H–M) Mid-segmentation stage wild-type (WT) and MZpnrc2 mutant embryos carrying the hsp70l:Venus-her1 3′UTRΔ1-546-SV40 pA reporter (line oz50) were heat shocked and processed by Venus in situ hybridization (n ≥ 7 embryos per time point). Venus transcript is not detected in the absence of heat shock (n = 10 wild-type embryos) (data not shown). Representative embryos were genotyped post-imaging to confirm genotype. For each reporter, three independent lines were analyzed in wild-type embryos by in situ hybridization and exhibited comparable Venus decay across all lines carrying the same reporter (data not shown); one representative line for each is shown (see Methods for details). pHS = post-heat shock; t1/2 = half-life; ± = standard deviation; pA = polyadenylation sequence. |
|
Fig. 4. Transgenic reporters reveal that the terminal her1 3′UTR is necessary and sufficient to confer transcript destabilization. Summary of reporter destabilization in transgenic embryos carrying various derivatives of the her1 3′UTR (A–E). All lines were raised to mid-segmentation stage and heat shocked for 15 min, then collected and processed by Venus in situ hybridization at 0, 30, 60, and 90 min pHS. (A) hsp70l:Venus-her1 3′UTR-SV40 pA reporter line (see Fig. 2, Fig. 6). (B) hsp70l:Venus-her1 3′UTRΔ1-362-SV40 pA reporter line (see Fig. 3). (C) hsp70l:Venus-her1 3′UTRΔ363-725-SV40 pA reporter line (see Fig. 3). (D) hsp70l:Venus-her1 3′UTRΔ1-546-SV40 pA reporter line (see Fig. 3). (E) hsp70l:Venus-her1 3′UTRΔ1-362;Δ547-725-SV40 pA reporter line (data not shown). Three independent lines were analyzed in wild-type embryos by in situ hybridization for each reporter, except for the hsp70l:Venus-her1 3′UTRΔ1-362;Δ547-725-SV40 pA reporter (E) for which two independent lines were analyzed. Each reporter exhibited comparable Venus decay across all independent lines (data not shown); see Methods for details. pHS = post-heat shock. |
|
Fig. 5. The dlc 3′UTR confers Pnrc2-mediated decay to reporter transcripts. (A–C) Transgenic embryos carrying the hsp70l:Venus-dlc 3′UTR-SV40 pA reporter (line oz81) were raised to mid-segmentation stage and heat shocked for 15 min, then collected at the indicated minutes pHS and processed by Venus in situ hybridization (n ≥ 6 embryos per time point). Venus transcript is not detected in the absence of heat shock (n = 10 embryos) (data not shown). (D–I) Mpnrc2 (D–F) and MZpnrc2 mutant embryos (G–I) carrying the hsp70l:Venus-dlc 3′UTR-SV40 pA reporter (line oz81) were also heat shocked and processed for Venus transcript (n ≥ 10 embryos per time point). Representative embryos were genotyped post-imaging to confirm genotype. (J) RBPmap motif analysis (Paz et al., 2014) identifies a canonical PRE (5′UGUAAAUA; yellow) and a canonical ARE (5′UAUUUAU; white) near the end of the her1 3′UTR and two PREs and one ARE near the end of the dlc 3′UTR. The indicated PRE and ARE are the only such motifs in the 725 nt full-length her1 3′UTR, whereas the 1327 nt dlc 3′UTR contains an additional PRE and two additional AREs. Three independent lines carrying the hsp70l:Venus-dlc 3′UTR-SV40 pA reporter were analyzed in wild-type embryos by in situ hybridization and exhibited comparable Venus decay across all three lines (data not shown); one representative line is shown (see Methods for details). pHS = post-heat shock; nts = nucleotides; PRE = Pumilio response element; ARE = AU-rich element; pA = polyadenylation sequence. |
|
Fig. 6 Download : Download high-res image (509KB)Download : Download full-size image Fig. 6. The Pumilio response and AU-rich elements in the her1 3′UTR contribute to reporter transcript turnover. (A–F) Transgenic embryos carrying the hsp70l:Venus-her1 3′UTR-SV40 pA reporter (line oz44) or the hsp70l:Venus-her1 3′UTR with disrupted PRE-SV40 pA reporter (line oz71) with a 2 nt mutation in the PRE sequence were raised to mid-segmentation stage and heat shocked for 15 min, then collected at the indicated minutes pHS and processed by Venus in situ hybridization (n ≥ 11 embryos per time point). Venus transcript is not detected in the absence of heat shock (n = 10 per reporter line) (data not shown). (G) qPCR analysis comparing Venus transcript fold change from 30 min pHS to 60 and 90 min pHS for the reporter lines Tg(hsp70l:Venus-her1 3′UTR-SV40 pA)oz44, Tg(hsp70l:Venus-her1 3′UTR with disrupted PRE-SV40 pA)oz71, and Tg(hsp70l:Venus-her1 3′UTR with disrupted ARE-SV40 pA)oz75 (n = 10 embryos per time point across three biological replicates). The PRE mutation changes the 5′UGUAAAUA site to 5′UCCAAAUA and the ARE mutation changes the 5′UAUUUAU site to 5′UACCCAU. Both mutations extend reporter half-life; the PRE-mutated reporter half-life is increased 1.7-fold and the ARE-mutated reporter half-life is increased 1.6-fold. Five independent lines carrying the hsp70l:Venus-her1 3′UTR with disrupted PRE-SV40 pA reporter and four independent lines carrying the hsp70l:Venus-her1 3′UTR with disrupted ARE-SV40 pA reporter were analyzed in wild-type embryos by in situ hybridization and exhibited comparable Venus decay across all lines carrying the same reporter (data not shown); one representative line for each is shown (see Methods for details). For each reporter, three independent lines were chosen for qPCR analysis and exhibited comparable Venus decay (Supplemental Fig. 3, Supplemental Fig. 4). pHS = post-heat shock; PRE = Pumilio response element; ARE = AU-rich element; t1/2 = half-life; ± = standard deviation; pA = polyadenylation sequence. |
|
Fig. 7. The Pumilio response and AU-rich elements in the her1 3′UTR are both required for rapid reporter transcript turnover. (A–C) Transgenic embryos carrying the hsp70l:Venus-her1 3′UTR with disrupted PRE & ARE-SV40 pA reporter (line oz93) with a 2 nt mutation in the PRE sequence and 3 nt mutation in the ARE sequence were raised to mid-segmentation stage and heat shocked for 15 min, then collected at the indicated minutes pHS and processed by Venus in situ hybridization (n ≥ 11 embryos per time point). Venus transcript is not detected in the absence of heat shock (n = 10 embryos) (data not shown). (D) qPCR analysis comparing Venus transcript fold change from 30 min pHS to 60 and 90 min pHS for the reporter lines Tg(hsp70l:Venus-her1 3′UTR-SV40 pA)oz44 and Tg(hsp70l:Venus-her1 3′UTR with disrupted PRE & ARE-SV40 pA)oz93 (n = 10 embryos per time point across three biological replicates). The presence of both mutations extends reporter half-life by ~7-fold compared to the unmutated control. Three independent lines carrying the hsp70l:Venus-her1 3′UTR with disrupted PRE & ARE-SV40 pA reporter were analyzed by in situ hybridization and qPCR and each line exhibited comparable Venus decay dynamics (data not shown and Supplemental Fig. 5); one representative line is shown (see Methods for details). pHS = post-heat shock; PRE = Pumilio response element; ARE = AU-rich element; t1/2 = half-life; ± = standard deviation; pA = polyadenylation sequence. |
Reprinted from Developmental Biology, 462, Tietz, K.T., Gallagher, T.L., Mannings, M.C., Morrow, Z.T., Derr, N.L., Amacher, S.L., Pumilio response and AU-rich elements drive rapid decay of Pnrc2-regulated cyclic gene transcripts, 129-140, Copyright (2020) with permission from Elsevier. Full text @ Dev. Biol.