- Title
-
Rbm24a Is Necessary for Hair Cell Development Through Regulating mRNA Stability in Zebrafish
- Authors
- Zhang, Y., Wang, Y., Yao, X., Wang, C., Chen, F., Liu, D., Shao, M., Xu, Z.
- Source
- Full text @ Front Cell Dev Biol
|
|
|
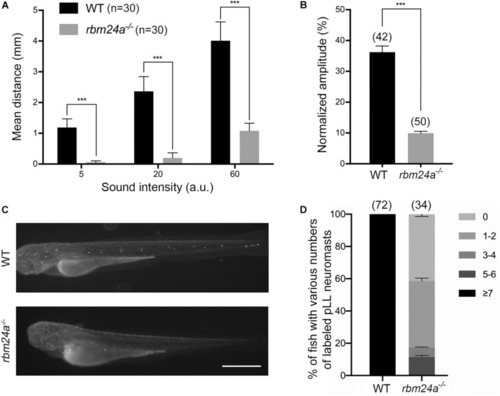
Hair cell function is affected in |
|
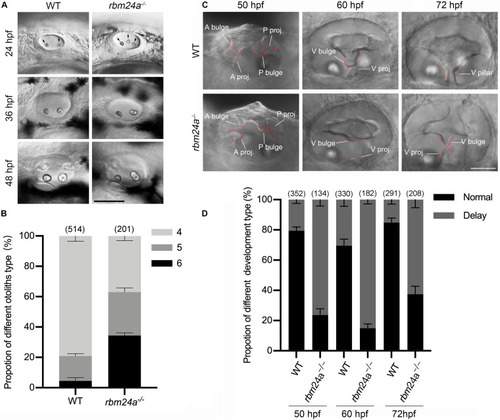
Otolith formation and semicircular canal fusion are delayed in |
|
Early inner ear development is largely unaffected in |
|
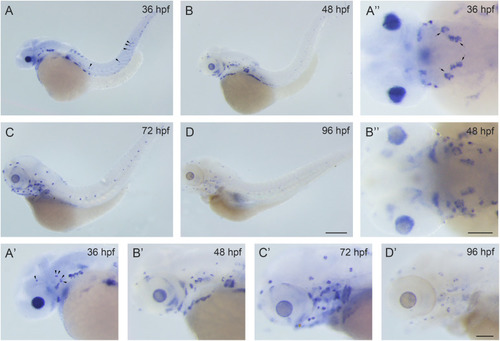
Development of pLL hair cells is affected in |
|
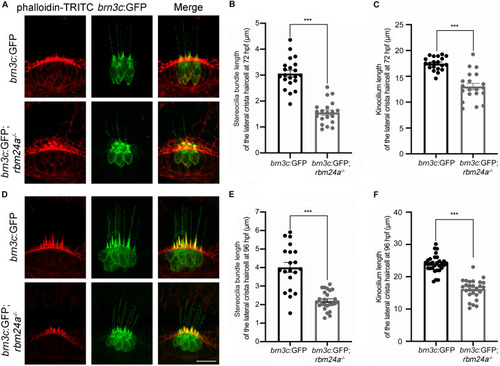
Development of lateral crista hair cells is affected in |
|
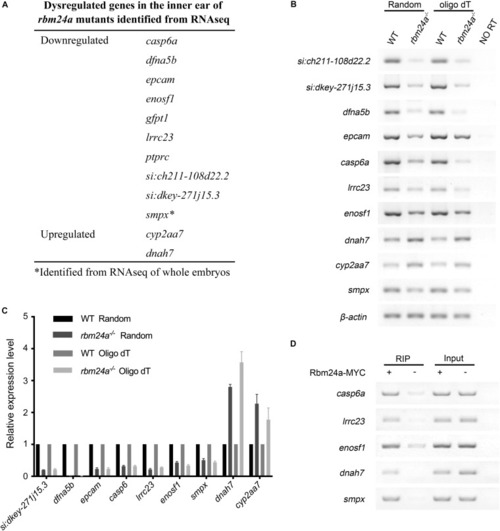
Dysregulated genes in the inner ear of |
|
Expression pattern of important Rbm24a target mRNAs in the zebrafish. |