- Title
-
Disruption of Abcc6 Transporter in Zebrafish Causes Ocular Calcification and Cardiac Fibrosis
- Authors
- Sun, J., She, P., Liu, X., Gao, B., Jin, D., Zhong, T.P.
- Source
- Full text @ Int. J. Mol. Sci.
|
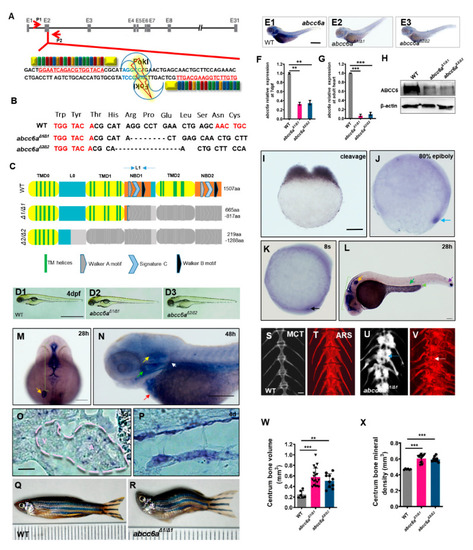
Generation of |
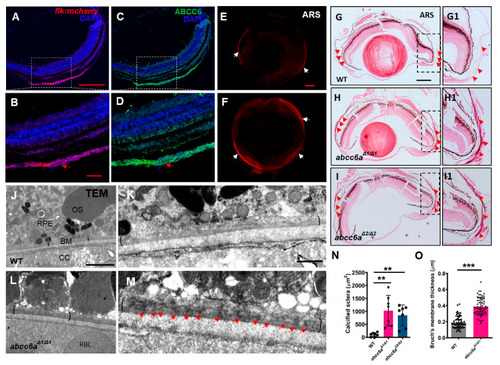
|
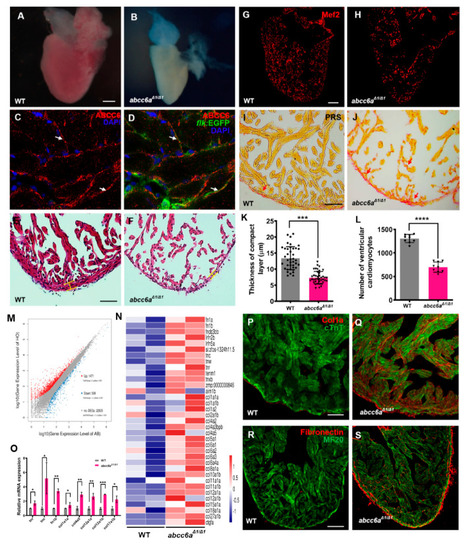
|
|
|
|
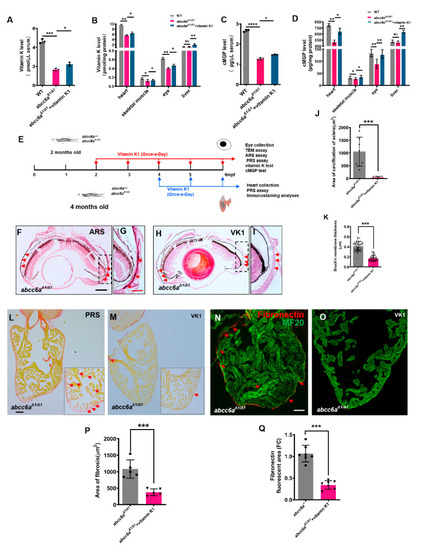
Vitamin K treatment relives ocular calcification and cardiac fibrosis in EXPRESSION / LABELING:
PHENOTYPE:
|