- Title
-
Circadian regulation of muscle growth independent of locomotor activity
- Authors
- Kelu, J.J., Pipalia, T.G., Hughes, S.M.
- Source
- Full text @ Proc. Natl. Acad. Sci. USA
|
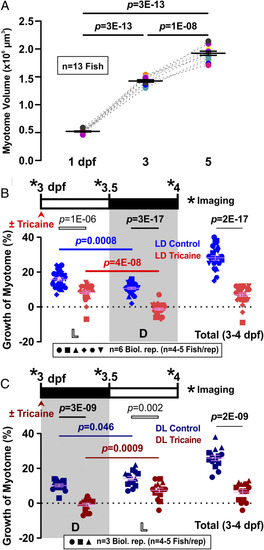
Diurnal variation and requirement for activity in volumetric growth of the myotome. ( |
|
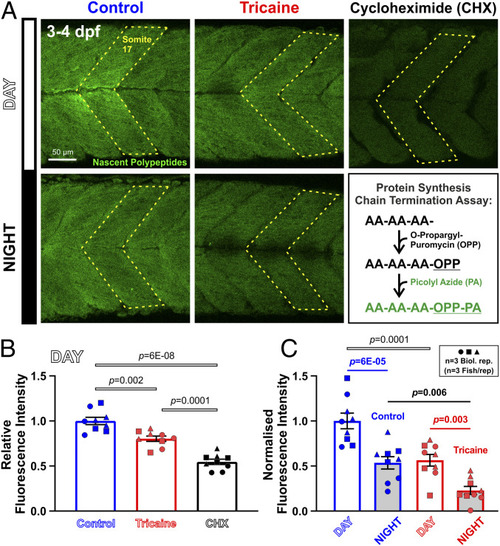
Diurnal variation and effect of activity in muscle protein synthesis. Larvae raised under LD that were either untreated (Control, blue) or anesthetized (Tricaine, red) at 3 dpf, and then treated with OPP for 2 h from ZT0 to ZT2 (Day) or ZT12 to ZT14 (Night). ( |
|
TORC1 signaling varies diurnally and is required for activity-driven daytime growth. Larvae raised under LD that were either untreated (Control, blue) or anesthetized (Tricaine, red) from 3 dpf to 4 dpf. ( |
|
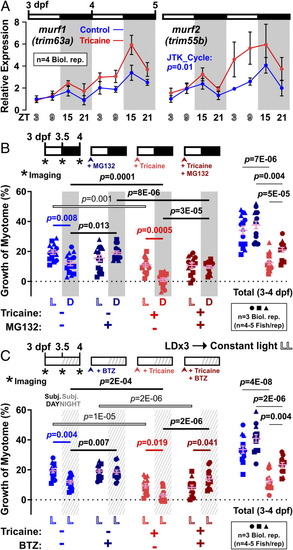
Nocturnal peak in atrogene expression parallels enhanced muscle growth at night in response to proteasome inhibitors. Larvae raised under LD were either untreated (Control, blue) or anesthetized (Tricaine, red). ( |
|
Circadian variation in muscle growth and metabolism is required for volumetric growth of myotome. ( |
|
Dominant negative clock (ΔCLK) reduces daytime and enhances nighttime growth of the myotome. Control |
|
Effects of ΔCLK expression on protein metabolism in zebrafish larvae. Control (light blue) and ΔCLK (dark blue) larvae were raised under LD and analyzed between 3 and 4 dpf. ( |
|
Models of how balance of anabolism and catabolism yields observed muscle growth through interaction of circadian and physical activity-dependent regulation. ( |