- Title
-
The second pharyngeal pouch is generated by dynamic remodeling of endodermal epithelium in zebrafish
- Authors
- Okada, K., Takada, S.
- Source
- Full text @ Development
|
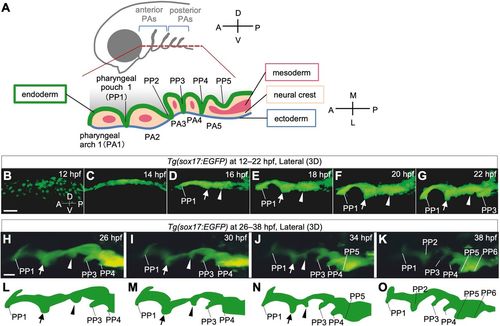
Time-lapse observations of pharyngeal endoderm during PP segmentation in Tg(sox17:EGFP) zebrafish embryos. (A) Schematic of the bilateral arrangement of PAs in the ventral region of the head. (B-K) Time-lapse analysis of the pharyngeal endoderm of Tg(sox17:EGFP) zebrafish from 12 hpf to 22 hpf (B-G, Movie 1) and from 26 hpf to 38 hpf (H-K, Movie 2). Rostral (arrows) and caudal (arrowheads) bulges appeared posterior to PP1 and gradually fused to form PP2. (L-O) Schematic illustrations of the shape of the lateral pharyngeal endoderm in H-K, respectively. A, anterior; D, dorsal; L, lateral; M, medial; P, posterior; PA1-5, the first to fifth pharyngeal arch; PP1-6, the first to sixth pharyngeal pouches; V, ventral. Scale bars: 50 μm (B) and 20 μm (H). EXPRESSION / LABELING:
PHENOTYPE:
|
|
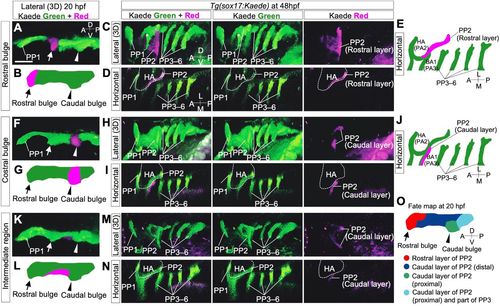
Lineage tracing of endodermal cells in Tg(sox17:Kaede) zebrafish embryos by photoconversion. (A-E) Cells of the rostral bulge (arrows) were marked at 20 hpf (A,B). At 48 hpf, cells of the rostral bulge contributed to the large area of the rostral portion of PP2 (C-E). (F-J) The cells of the caudal bulge (arrowheads) were marked at 20 hpf (F,G). At 48 hpf, the descendant cells contributed to the caudal region of PP2 rather proximally (H-J). (K-N) The cells of the intermediate domain of a putative PP2 (between the rostral and caudal bulges) were marked (K,L). At 48 hpf, these descendants composed the dorsally and ventrally distant area in the caudal part of PP2 (M,N). (O) Overview of the cell fate of future PP2 endoderm at 20 hpf. Cell fates were examined in various regions of the presumptive PP2 endoderm by photoconversion (n=29, A-N and Fig. S1); these are summarized, showing the dynamic reorganization of the endoderm forming PP2. A, anterior; BA, branchial arch; D, dorsal; HA, hyoid arch; L, lateral; M, medial; P, posterior; PP1-6, the first to sixth pharyngeal pouches; V, ventral. The asterisk indicates a blood vessel. The positions of the HA (dashed lines) were identified by the surrounding sox17-positive endoderm. Scale bar: 50 μm. PHENOTYPE:
|
|
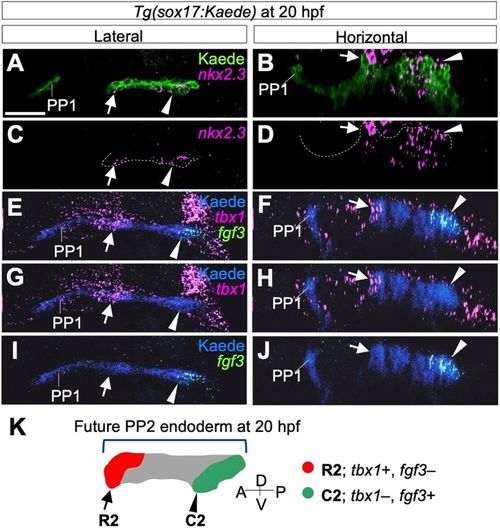
Separate formations and rostrocaudal identity of PP2 endoderm at 20 hpf. (A-D) Separate expression of nkx2.3 indicated the rostral (arrows) and caudal (arrowheads) bulges of PP2 endoderm identified immunohistochemically with the anti-Kaede antibody. (E-J) Expression of tbx1 (E-H) and fgf3 (E,F,I,J) was detected in rostral (arrows) and caudal (arrowheads) bulges of PP2, showing early specification of the rostrocaudal polarity of PP2. (K) According to fate analysis and molecular profiles, the rostral and the caudal bulges of the future PP2 are distinctly defined as R2 (red, arrow) and C2 (green, arrowhead), respectively. Whereas cells of the intermediate region (gray) contribute to the caudal region, fgf3, a caudal marker, is not expressed in these cells at 20 hpf. A, anterior; D, dorsal; P, posterior; PP1, the first pharyngeal pouch; V, ventral. Scale bar: 20 μm. Four embryos were analyzed for nkx2.3 expression and three embryos for tbx1 and fgf3 expression. EXPRESSION / LABELING:
|
|
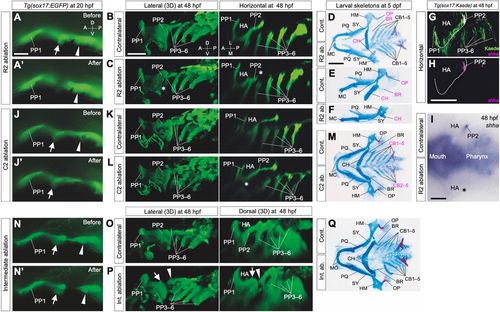
Early determination of distinct roles for later skeletal patterns in R2 and C2 endoderm. (A,A′) Cells of R2 (arrows) in Tg(sox17:EGFP) embryos were ablated at 20 hpf. (B-F) R2 ablation caused a specific loss of epithelial expansion of the caudal lining of the HA (asterisks in C; n=3/3) and reductions in HA-derived skeletal elements, especially in the opercular series (D-F; OP, n=10/16; BR, n=12/16). In addition, this ablation occasionally caused a size reduction in other HA-derived skeletal structures, such as the HM (Fig. S3C; n=3/16) and CH (D-F; n=6/16). (G,H) Expression of shha, required for opercular development, was detected in PP2 endoderm occupied by R2 descendants. (I) Consistent with endodermal (B,C) and the skeletal (D-F) phenotypes, R2 ablation caused a specific loss of shha expression in PP2, as shown in a flat-mounted embryo (asterisk in I; n=12). (J,J′) Cells of C2 (arrowheads) in Tg(sox17:EGFP) embryos were ablated at 20 hpf. (K-M) Ablation of C2 cells resulted in loss of the proximal region of PP2, which consists of the rostral lining of the third PA (BA1) (K,L, asterisk in L; n=3/3), resulting in loss of CB1 cartilage (M; n= 8/8). (N,N′) Endodermal cells between R2 (arrows) and C2 (arrowheads) were ablated in Tg(sox17:EGFP) embryos at 20 hpf. (O-Q) Ablation of cells in the intermediate region did not affect segregation of HA and BA1 but caused abnormal arrangements of them, shown by a split between HA and BA1 (O,P; n=10/12). Correspondingly, on the ablated sides, the positions of BA1-derived CB1 cartilage shifted posteriorly, although a complete set of the pharyngeal structures developed (Q; n=4/6). Images of ablated sides (C,F,L,Q) are inverted in a left-right direction for comparisons with contralateral sides. A, anterior; BR, branchiostegal ray; CB1-5, the first to fifth ceratobranchials; CH, ceratohyal cartilages; D, dorsal; HA, hyoid arch; HM, hyomandibular; L, lateral; M, medial; MC, Meckel's cartilage; OP, opercular bone; P, posterior; PP1-6, the first to sixth pharyngeal pouches; PQ, palatoquadrate; SY, symplectic; V, ventral. Scale bars: 20 μm (A), 50 μm (B,H,I) and 100 μm (D). EXPRESSION / LABELING:
PHENOTYPE:
|
|
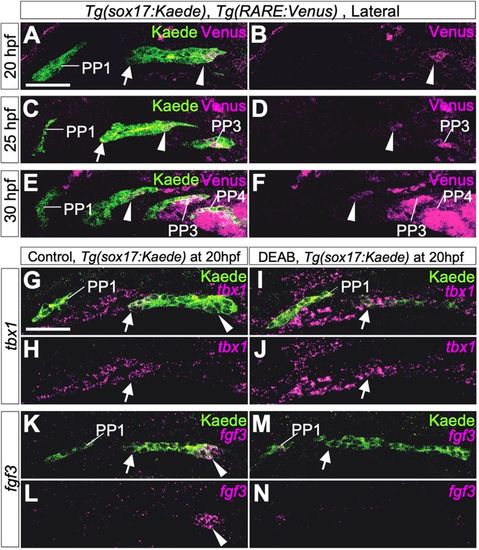
Boundaries of molecular mechanisms forming PPs between the rostral and caudal portions of PP2. (A-F) Immunohistochemistry of double transgenic embryos of Tg(sox17:Kaede) and Tg(RARE:Venus) showed specific signals of the RA reporter Venus in the caudal part of PP2 and in posterior PPs but not in the rostral edge of PP2 and PP1 endoderm at 20 hpf (A,B), 25 hpf (C,D) and 30 hpf (E,F). Three embryos were analyzed at each time point. (G-N) Expression of tbx1 (magenta in G-J) or fgf3 (magenta in K-N) in control (G,H,K,L) and DEAB-treated transgenic (I,J,M,N) embryos carrying sox17:Kaede R2 (arrows) at 20 hpf. Expression of tbx1 was not affected in DEAB-treated embryos (G,J, indicated by arrows). By contrast, RA deficiency caused by DEAB treatment resulted in loss of fgf3 expression in C2 (K-N, indicated by arrowheads). Owing to the angle at which images were taken, the size and morphology of each PP appear to be slightly different in each confocal plane, but, in these experiments, DEAB treatment did not cause major anomalies in morphology of PP1 or PP2. More than five embryos were used for each experiment. EXPRESSION / LABELING:
|
|
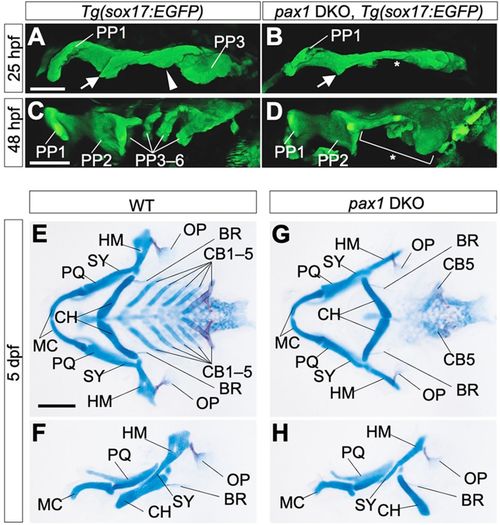
Pharyngeal abnormalities in pax1 dKO mutant embryos. (A-D) Endodermal morphologies of wild-type (A,C) and pax1a; pax1b double-knockout (pax1 DKO) embryos (B,D) harboring a Tg(sox17:EGFP) transgene. At 25 hpf, PP1, R2, C2 and PP3 were formed in the wild type (A), but, in pax1 DKO embryos, C2 and PP3 were specifically defective (B, asterisk). At 48 hpf, complete segments of PP were observed in the wild type (C), whereas caudal PP2 and more posterior PPs were not formed in pax1 DKO embryos (D, bracket and asterisk). Notably, PP1 and the rostral part of PP2 were almost normal in the mutants (D). All pictures show the left side of the pharyngeal region. PP1-6, the first to sixth pharyngeal pouches. Arrows indicate R2; arrowheads indicate C2. Scale bars: 50 μm. (E-H) Flat-mount views of pharyngeal skeletons (E,G) and left-side views of MA- and HA-derived skeletal elements (F,H) in wild-type (E,F) and pax1 DKO (G,H) larvae at 5 days post-fertilization (dpf). CB1-4 and the anterior part of the HM were lost, although OP and BR were normally formed in pax1 DKO larvae (G,H). BR, branchiostegal ray; CB1-5, the first to fifth ceratobranchials; CH, ceratohyal; HM, hyomandibular; MC, Meckel's cartilage; OP, opercular bone; PQ, palatoquadrate; SY, symplectic. Scale bars: 100 μm. Three embryos were used for endoderm observation and five embryos were used for skeletal analysis. |
|
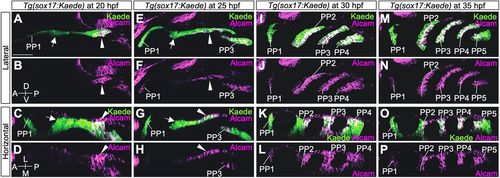
Expression analysis of Alcam in the PP endoderm of Tg(sox17:Kaede). (A-D) At 20 hpf, strong expression of Alcam was evident in C2 but hardly detected in PP1 and R2 endoderm. (E-H) At 25 hpf, Alcam was high in PP3 and the caudal portion of PP2 but almost absent in PP1 and the rostral part of PP2. (I-L) At 30 hpf, high accumulation of Alcam was detected in PP3, PP4 and the caudal layer of PP2, whereas it was at a very low level in PP1 and the rostral layer of PP2. (M-P) At 35 hpf, high accumulation of Alcam was detected in PP3, PP4, PP5 and the caudal part of PP2, whereas it was at a very low level in PP1 and the rostral layer of PP2. A, anterior; D, dorsal; L, lateral; M, medial; P, posterior; PP1-5, the first to fifth pharyngeal pouches; V, ventral. Arrows indicate R2; arrowheads indicate C2. Scale bar: 50 μm. More than two embryos were analyzed at each time point. EXPRESSION / LABELING:
PHENOTYPE:
|