- Title
-
Single-cell transcriptomic analysis identifies the conversion of zebrafish Etv2-deficient vascular progenitors into skeletal muscle
- Authors
- Chestnut, B., Casie Chetty, S., Koenig, A.L., Sumanas, S.
- Source
- Full text @ Nat. Commun.
|
|
|
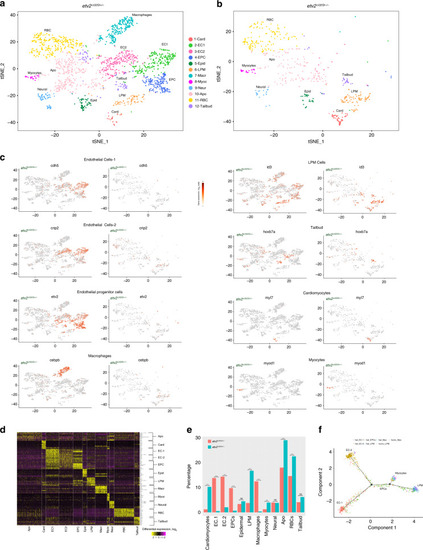
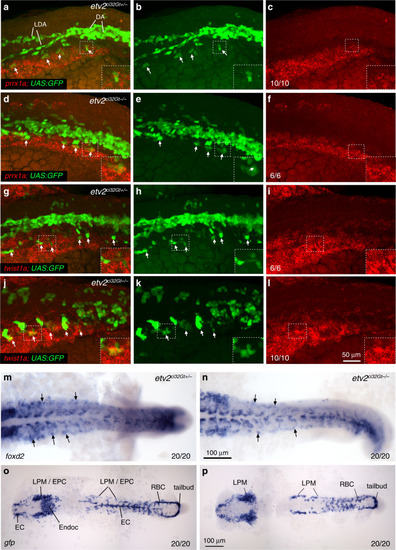
Subclustering of the EC1 population in EXPRESSION / LABELING:
|
|
EXPRESSION / LABELING:
|
|
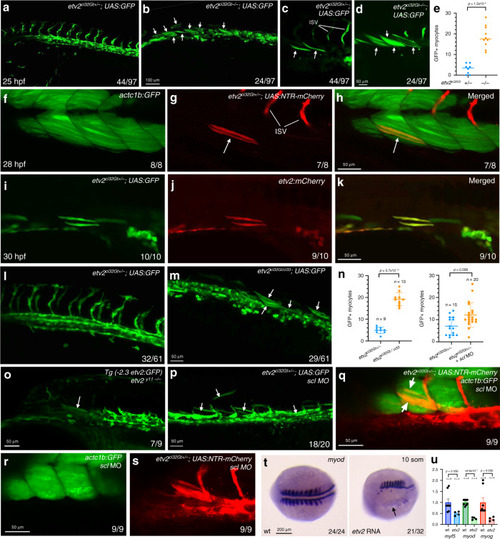
Lateral view is shown, anterior is to the left. |
|
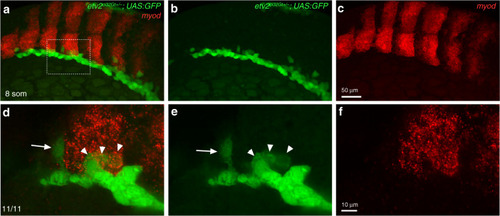
In situ hybridization was performed using hybridization chain reacion (HCR) at the 8–10-somite stages. EXPRESSION / LABELING:
PHENOTYPE:
|
|
PHENOTYPE:
|
|
EXPRESSION / LABELING:
|
|
EXPRESSION / LABELING:
|
|
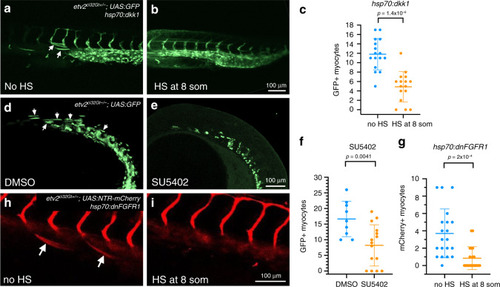
Wnt and FGF signaling promotes myocyte differentiation of multipotent progenitors in the lateral plate mesoderm (LPM). BMP signaling through its downstream effectors |