- Title
-
Targeted cell ablation in zebrafish using optogenetic transcriptional control
- Authors
- Mruk, K., Ciepla, P., Piza, P.A., Alnaqib, M.A., Chen, J.K.
- Source
- Full text @ Development
|
M2H37A channel variant is toxic to zebrafish embryos and ablates neurons more rapidly than NTR. (A) Wild-type zygotes were injected with the designated amount of M2H37A mRNA and cultured in the absence or presence of 100 μg/ml rimantadine. Representative bright-field micrographs of 24 hpf embryos from two independent experiments are shown. Scale bar: 500 μm. (B) Phenotypic distributions of embryos injected with the designated amount of M2H37A mRNA. The embryos were scored at 24 hpf, and the number of embryos (n) per condition from two or three independent experiments is indicated. Statistical analyses: χ2=389, d.f.=14, P<0.00001. (C) Comparison of Tg(tuba1a:Gal4VP16;myl7:GFP) and Tg(tuba1a:Gal4VP16; UAS:M2H37A;myl7:gfp,mCherry) embryos cultured in the absence or presence of 100 µg/ml rimantadine. Neuronal expression of M2H37Ainduces loss of the midbrain-hindbrain boundary (arrowheads) at 24 hpf and increasingly severe CNS deficits as development continues. Treatment with rimantadine starting at 10 hpf rescues these neuronal defects in Tg(tuba1a:Gal4VP16;UAS:M2H37A;myl7:gfp,mCherry)embryos. Representative bright-field and epifluorescence micrographs (overlays) from three independent experiments are shown. (D) Comparison of Tg(tuba1a:Gal4VP16;UAS:NTR-mCherry;myl7:GFP) embryos cultured with 5 mM metronidazole or an equivalent amount of DMSO, starting at 10 hpf. Metronidazole-treated embryos first exhibit CNS defects at 48 hpf. Representative bright-field and epifluorescence micrographs (overlays) from two independent experiments are shown. All embryos are shown in lateral view, anterior left. Scale bars: 250 µm. Statistics for the observed phenotypes are in Table S1. |
|
GAVPO conveys light-inducible gene expression in zebrafish. (A) Mechanism of GAVPO-dependent transcription of a transgene with an upstream activating sequence (UAS) and minimal promoter (MP). (B) Tg(UAS:NTR-mCherry) zygotes were injected with the designated amounts of GAVPO mRNA, irradiated with a blue LED lamp at 6 hpf, and then imaged at 24 hpf. Representative bright-field and epifluorescence micrographs from two to four independent experiments are shown. GAVPO induces NTR-mCherry expression in a light- and concentration-dependent manner. Scale bar: 250 µm. (C) Quantification of mCherry fluorescence in Tg(UAS:NTR-mCherry)zygotes were injected with the designated amounts of GAVPOmRNA, as described in B. The box extends from the 25th to 75th percentiles. The whiskers extend to the minimum and maximum values. The horizontal line indicates the average value. (D) The designated amounts of GAVPO or Gal4VP16 mRNA were injected into Tg(UAS:NTR-mCherry) zygotes, and the embryos were either cultured in the dark or globally exposed to blue LED light (3 mW/cm2) from 6 to 8 hpf. Developmental phenotypes were then scored at 24 hpf, using the indicated number of embryos (n) per condition from two to four independent experiments. Statistical analyses: χ2=190, d.f.=14, P<0.00001. Statistics for the observed phenotypes are in Table S2. |
|
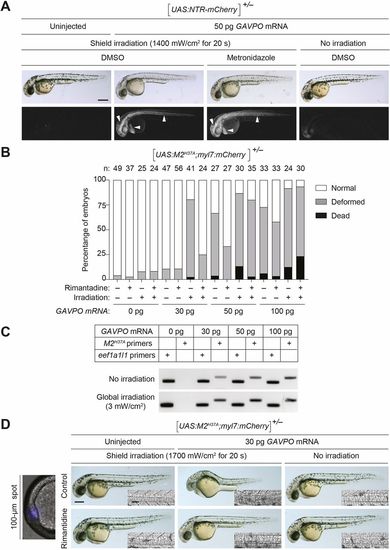
GAVPO-mediated toxicity in UAS:NTR-mCherry and UAS:M2H37A zebrafish lines. (A) Tg(UAS:NTR-mCherry) zygotes were injected with the designated amounts of GAVPO mRNA, and their embryonic shields were irradiated at 6 hpf using an epifluorescence microscope equipped with a 470/40 nm filter. An iris diaphragm was used to restrict illumination to a 100 µm diameter region. The 10 hpf embryos were dechorionated and treated with DMSO or 5 mM metronidazole, and imaged at 32 hpf. Representative bright-field and epifluorescence micrographs from two independent experiments are shown. Shield-irradiation of the GAVPO-expressing embryos induces NTR-mCherry expression in the notochord, head mesoderm and hatching gland (arrowheads). (B) The designated amounts of GAVPO mRNA were injected into Tg(UAS:UAS:M2H37A; myl7:mCherry) zygotes, and the embryos were either cultured in the dark or globally exposed to blue LED light (3 mW/cm2) from 6 to 8 hpf and then cultured in the absence or presence of 100 μg/ml rimantadine. Developmental phenotypes were scored at 24 hpf, using the indicated number of embryos (n) per condition from two to four independent experiments. Statistical analyses: χ2=301, d.f.=30, P<0.00001. (C) RT-PCR analyses of M2H37A expression in Tg(UAS:UAS:M2H37A;myl7:mCherry) embryos injected with GAVPOmRNA and treated as described above. The expression of the translation elongation factor eef1a1l1 was used as a normalization control. The amplification reaction was performed using a standard PCR with 40 cycles for M2 and 35 cycles for eef1a1l1 amplification. (D) Tg(UAS:M2H37A;myl7:mCherry) zygotes were injected with the designated amounts of GAVPO mRNA, cultured in the absence or presence of 100 µg/ml rimantadine, shield-irradiated at 6 hpf as described in A, and imaged at 30 hpf. Representative bright-field and differential interference contrast (DIC) micrographs from three independent experiments are shown. Shield irradiation of the GAVPO-expressing embryos disrupts development of the notochord (inset), head and hatching gland. All embryos are shown in lateral view, anterior left. Scale bars: 250 µm in A,D; 50 µm in D (insets). Statistics for the observed phenotypes are in Table S3. |
|
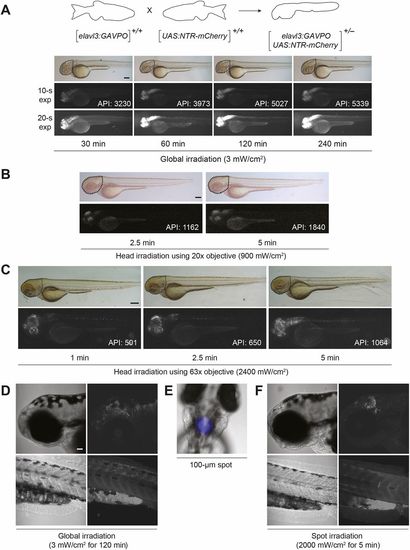
Light-inducible, neuron-specific gene expression using the GAVPO system. (A) Tg(elavl3:GAVPO;UAS:NTR-mCherry)embryos were irradiated for varying durations, using a blue LED lamp and starting at 48 hpf. The embryos were then imaged 8 h later, and representative bright-field and epifluorescence micrographs (with short or long exposure times) are shown. NTR-mCherry was expressed in neural tissues, at levels that increased with the length of irradiation. Dashed outlines indicate the region used to determine the average pixel intensity (API) for each micrograph. (B,C) The heads of 48 hpf Tg(elavl3:GAVPO;UAS:NTR-mCherry) embryos were irradiated using an epifluorescence microscope equipped with a 470/40 nm filter and either a 20× (B) or 63× (C) objective. The embryos were imaged 8 h later, and representative bright-field and epifluorescence micrographs are shown. NTR-mCherry expression was observed in anterior neural tissues, at levels that increased with the duration and intensity of irradiation. Dashed outlines indicate the region used to determine the average pixel intensity (API) for each micrograph. (D-F) Tg(elavl3:GAVPO;UAS:NTR-mCherry) embryos were illuminated either globally using a blue LED lamp (D) or within a 100 µm diameter region in the head using an epifluorescence microscope equipped with a 470/40 nm filter, 20× objective and iris diaphragm (E,F). Representative bright-field and confocal fluorescence micrographs from two independent experiments are shown, and total sample sizes are indicated. LED illumination induced NTR-mCherry expression throughout the head, whereas spot-irradiated embryos exhibited mCherry fluorescence in only a localized anterior region. Embryo orientations: A-D,F, lateral view and anterior left; E, dorsal view, anterior up. Scale bars: 200 µm in A; 300 µm in B,C; 50 µm in D-F. Statistics for the observed phenotypes are in Table S4. |
|
Light-inducible neural ablation using the GAVPO/M2H37Asystem. (A) Heterozygous Tg(elavl3:GAVPO;UAS:M2H37A;myl7:mCherry) embryos were injected with an elavl3:mCherry-CAAX construct to label neurons in a mosaic manner. The injected embryos were globally irradiated with a blue LED lamp at 48 hpf for 8 h, fixed 12 h later and then imaged using a confocal microscope. Representative micrographs from a hindbrain slice just above the otic vesicle are shown. Irradiated embryos have fewer intact neurons (arrowheads) than those cultured in the dark or treated with 100 µg/ml rimantadine during blue-light illumination. Neurons were counted in ImageJ in a blinded manner, and the average number of neurons per slice±s.d. for each experimental condition is shown. (B) Representative micrographs from a maximal intensity projection of the spinal cord just above the cloaca, with axonal tract widths in selected regions indicated by the dashed lines. The average axonal tract width±s.d. for each experimental condition are shown. Values for individual fish are shown. The box extends from the 25th to 75th percentiles. The whiskers extend to the minimum and maximum values. The horizontal line indicates the average value. Embryo orientations: A,B, lateral view, anterior left. Scale bars: 25 µm. |
|
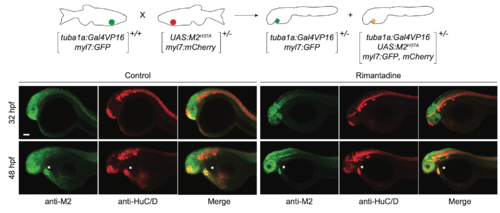
Neuron-specific expression of M2H37A. Representative maximum intensity projection micrographs of heterozygousTg(tuba1a:Gal4VP16;UAS:M2H37A;myl7:GFP, mCherry) embryos immunostained for the M2-derived channel (green) or HuC/D (red), an early neuronal marker. Asterisks denote residual fluorescence from reporter genes expressed in the heart. Embryo orientations: lateral view, anterior left. Scale bar: 100 μm. |
|
M2H37A- and NTR-induced cell death in zebrafish embryos. (A) Heterozygous Tg(tuba1a:Gal4VP16; UAS:M2H37A;myl7:GFP,mCherry) and Tg(tuba1a:Gal4VP16;UAS:NTR-mCherry;myl7:GFP) embryos were cultured in medium supplemented with DMSO, 100 μg/mL rimantadine, or 5 mM metronidazole at 10 hpf. At 48 hpf, the embryos were fixed, and apoptotic cells visualized by TUNEL staining. TUNEL-positive cells were limited to the CNS at 48 hpf in NTR-expressing embryos treated with metronidazole, but they were observed outside the CNS in M2H37A-express- ing embryos cultured without rimantadine. (B) Heterozygous Tg(tuba1a:Gal4VP16; UAS:M2H37A;myl7:GFP,mCherry) embryos cultured in the presence or absence of 100 μg/mL rimatidine, fixed, and immunostained for activaterd caspase-3 (CASP3). (C) Heterozygous Tg(UAS:NTR-mCherry) zygotes were injected with the designated amount of GAVPO mRNA, and their embryonic shields were irradiated at 6 hpf. The embryos were then cultured in the absence or presence of 5 mM metronidazole from 10 to 32 hpf, fixed, and immunostained for mCherry and activated CASP3 (arrowheads). GAVPO-, light-, and metronidazole-dependent caspase-3 activation was observed. Embryo orienta- tions: lateral view, anterior left. Scale bars: 300 μm. |
|
Optimization of light-inducible, neuron-specific gene expression using the GAVPO system. (A-B) Tg(elavl3:GAVPO; UAS:NTR-mCherry) embryos were irradiated globally for varying durations, starting at 48 hpf and using a (A) blue LED lamp or (B) white LED lamp. The embryos were imaged at 56 hpf, and representative brightfield and epifluorescence micrographs are shown. (C) Quantification of mCherry fluorescence in Tg(elavl3:GAVPO;UAS:NTR-mCherry) embryos irradiated as described in A-B. Embryo orientations:lateral view and anterior left. Scale bars: 500 μm. |
|
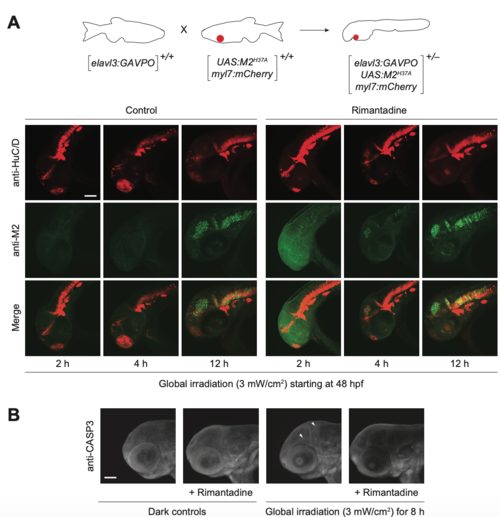
Light-inducible, neuron-specific M2H37A expression using the GAVPO system. (A) Tg(elavl3:GAVPO; UAS:M2H37A;myl7:mCherry) embryos were globally irradiated with blue LED light at 48 hpf for the designated durations and cultured in the presence or absence of rimantadine. The embryos were then fixed, and immunostained for M2 and HuC/D expression. Representative confocal micrographs are shown as maximum intensity projections of 39 to 51 z-stack images (5-μm optical sections), demonstrating overlaying domains of M2H37A and HuC/D expression in the anterior CNS. (B) Tg(elavl3:GAVPO;UAS:M2H37A;myl7:mCherry) embryos were globally irradiated with blue LED light at 48 hpf for 8 h and cultured in the presence or absence of rimantadine. The embryos were fixed 12 h later, and immunostained for activated caspase-3. Representative confocal micrographs are shown as maximum intensity projections of 30 to 50 z-stack images (7-μm optical sections), demonstrating limited non-cell autonomous apoptosis. Arrowheads: cells with activated caspase-3. Embryo orientations: lateral view, anterior left. Scale bars: 1-0 μm. |