- Title
-
Application of optogenetic Amyloid-β distinguishes between metabolic and physical damages in neurodegeneration
- Authors
- Lim, C.H., Kaur, P., Teo, E., Lam, V.Y.M., Zhu, F., Kibat, C., Gruber, J., Mathuru, A.S., Tolwinski, N.S.
- Source
- Full text @ Elife

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. |
|
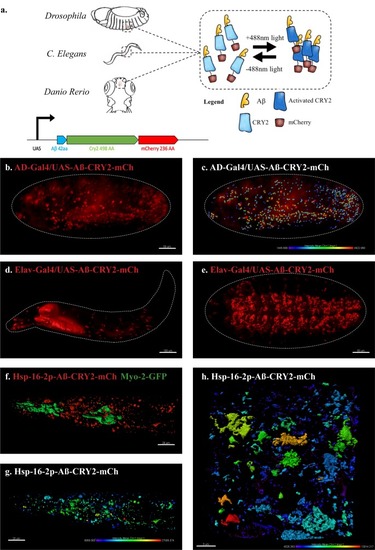
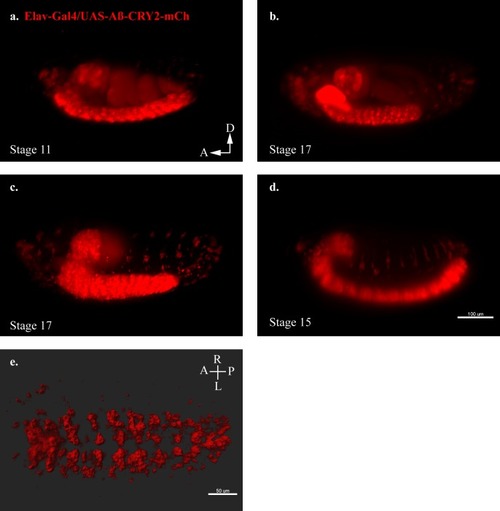
An in vivo, light-dependent, oligomerization switch for the formation or dissolution of Aβ aggregates in the fruit fly and nematode.(a) A schematic of the strategy and a relative size comparison of the three components of UAS-Aβ-CRY2-mCh. (b) Expression of AD-Gal4/UAS-Aβ-CRY2-mCh in a Drosophila embryo. (c) Mean intensity of aggregates in the same AD-Gal4/UAS-Aβ-CRY2-mChDrosophilaembryo. (d and e) Expression of Elav-Gal4/UAS-Aβ-CRY2-mCh in a Drosophila larva and embryo. (f) Expression of hsp-16–2 p-Aβ-CRY2-mCh in heat-shocked C. elegans with myo-2-gfp marker, that marks its pharyngeal pump and serves as an indicator for positive microinjection. (g and h) Mean intensity of aggregates in the hsp-16–2 p-Aβ-CRY2-mCh in C.elegans under 20x and 63x magnification of the confocal microscope; 63x image was processed using the Zeiss Airyscan. |
|
( |
|
( |
|
( |
|
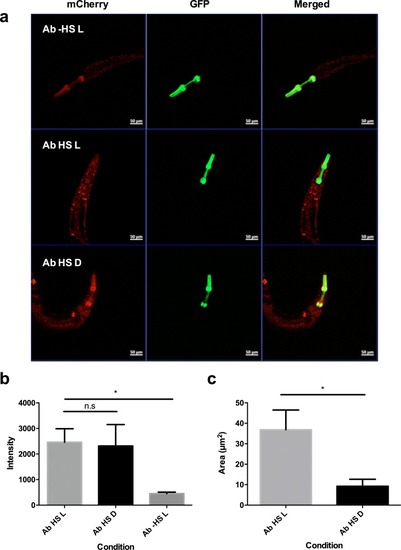
Embryos expressing Aβ-CRY2-mCh ubiquitously showed significantly lesser Aβ aggregates in the ( |
|
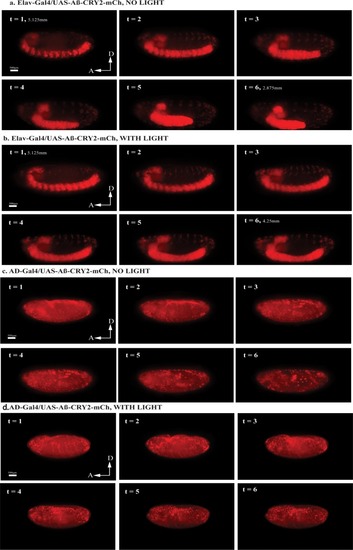
Images and percentage fluorescence recovery after bleaching of ( |
|
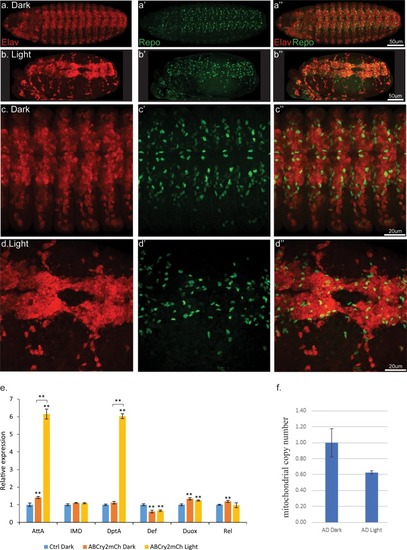
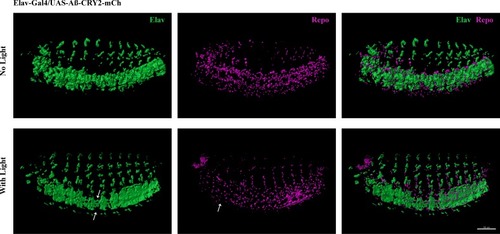
Light induced aggregation causes physical disruption of embryonic neural tissues.(a–a’’) Elav-Gal4/UAS-Aβ-CRY2-mCh embryos kept in the dark and imaged for neurons (Elav) and glial cells (Repo). (b–b’’) Elav-Gal4/UAS-Aβ-CRY2-mCh embryos exposed to light and imaged for neurons (Elav) and glial cells (Repo). (c–d’’) Close ups. (E) Aggregation induced inflammatory response as measured by qPCR for inflammatory response genes. (f) The number of mitochondria in transgenic Aβ Drosophila embryo was reduced by 40% in light. |
|
Reduction of neurons and glia cells (arrows) detected by immunostaining of Elav and Repo in transgenic Aβ |
|
( |
|
Schematic of lightsheet imaging/illumination lens orientation to |