- Title
-
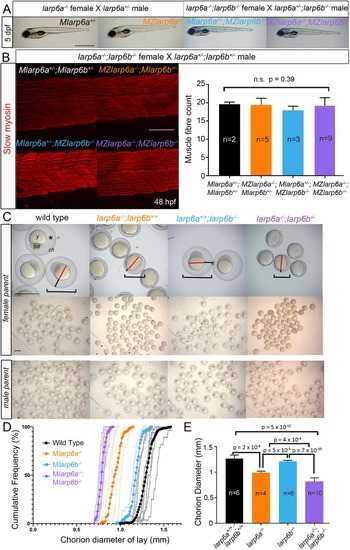
Maternal Larp6 controls oocyte development, chorion formation and elevation
- Authors
- Hau, H.T.A., Ogundele, O., Hibbert, A.H., Monfries, C.A.L., Exelby, K., Wood, N.J., Nevarez-Mejia, J., Carbajal, M.A., Fleck, R.A., Dermit, M., Mardakheh, F.K., Williams-Ward, V.C., Pipalia, T.G., Conte, M.R., Hughes, S.M.
- Source
- Full text @ Development
|
|
|
|
|
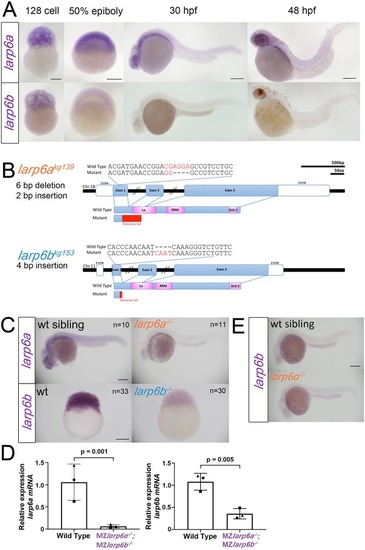
EXPRESSION / LABELING:
PHENOTYPE:
|
|
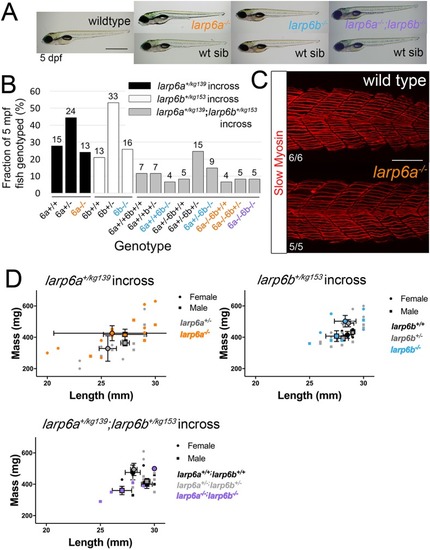
EXPRESSION / LABELING:
PHENOTYPE:
|
|
|
|
|