- Title
-
Dopaminergic Co-Regulation of Locomotor Development and Motor Neuron Synaptogenesis is Uncoupled by Hypoxia in Zebrafish
- Authors
- Son, J.H., Stevenson, T.J., Bowles, M.D., Scholl, E.A., Bonkowsky, J.L.
- Source
- Full text @ eNeuro
|
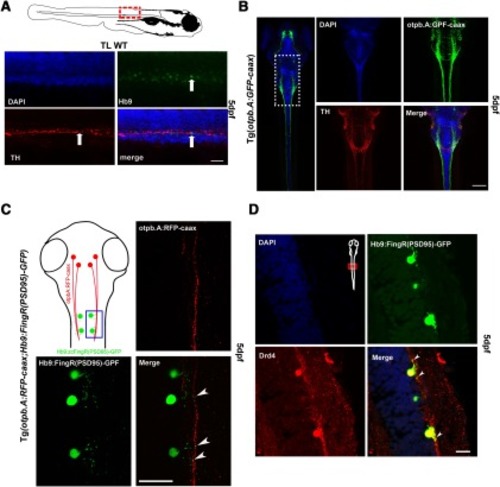
Dopaminergic synapses to spinal cord motor neurons. |
|
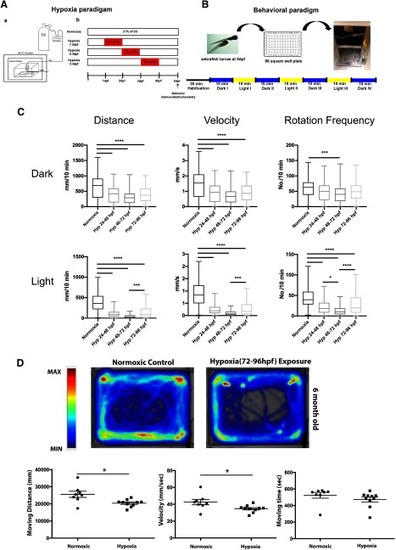
Hypoxia causes impaired motor behavior in larval and adult animals. |
|
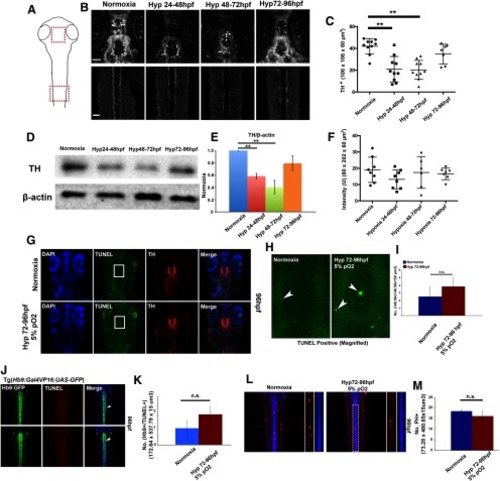
Hypoxia does not alter dopamine or motor neuron numbers, or axon pathfinding of the DDT after hypoxia from 3 to 4 dpf. |
|
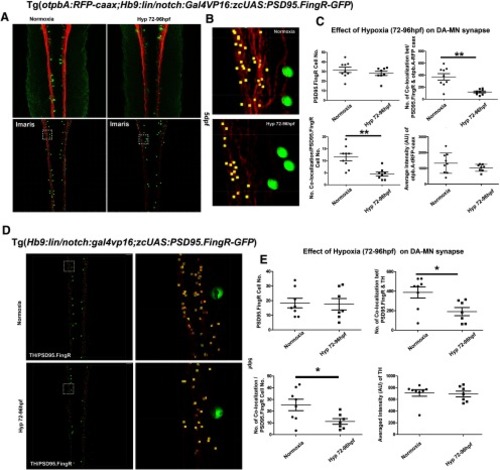
Hypoxia causes a decrease in DDT/motor neuron synapses. |
|
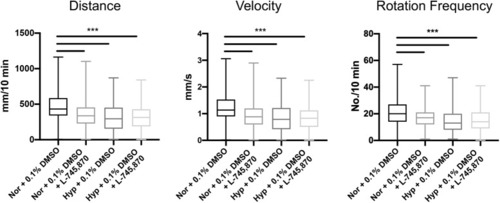
Dopaminergic antagonist exposure impairs zebrafish locomotor development similar to effects of hypoxia. Analysis of zebrafish larvae at 5 dpf, following exposure to L-745870 from 72 to 96 hpf. Impairments in distance, velocity, and rotation frequency (quantified data in |