- Title
-
Clinical and preclinical therapeutic outcome metrics for USH2A-related disease
- Authors
- Toms, M., Dubis, A.M., de Vrieze, E., Tracey-White, D., Mitsios, A., Hayes, M., Broekman, S., Baxendale, S., Utoomprurkporn, N., Bamiou, D., Bitner-Glindzicz, M., Webster, A.R., Van Wijk, E., Moosajee, M.
- Source
- Full text @ Hum. Mol. Genet.

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. |

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. |
|
Position and type of |
|
Visual acuity, EZ and hyperautofluorescent (hyperAF) ring measurements with age in |
|
Inter-relatedness of progression between metrics. While there is a strong correlation between the rate of change observed in the first and second observation periods for both EZ length constriction ( |
|
Degree and progression of hearing loss in |
|
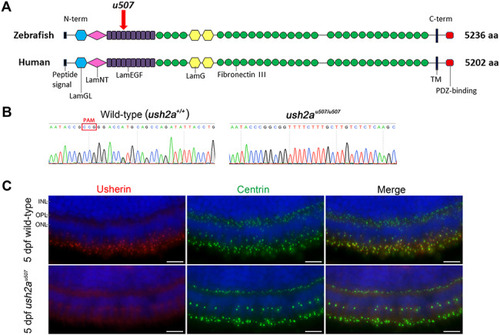
Generation of |
|
Visual function in |
|
Auditory function in |
|
Rod and cone opsin localization in the |
|
Retinal ultrastructure of PHENOTYPE:
|
|
Rhodopsin mislocalization and elevated autophagy in |