- Title
-
Midkine-a is required for cell cycle progression of Müller glia glia during neuronal regeneration in the vertebrate retina
- Authors
- Nagashima, M., D'Cruz, T.S., Danku, A.E., Hesse, D., Sifuentes, C., Raymond, P.A., Hitchcock, P.F.
- Source
- Full text @ J. Neurosci.
|
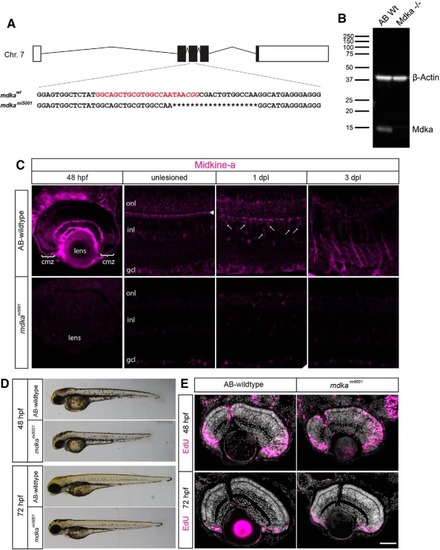
Midkine-a governs cell cycle kinetics during retinal development. |
|
In the PHENOTYPE:
|
|
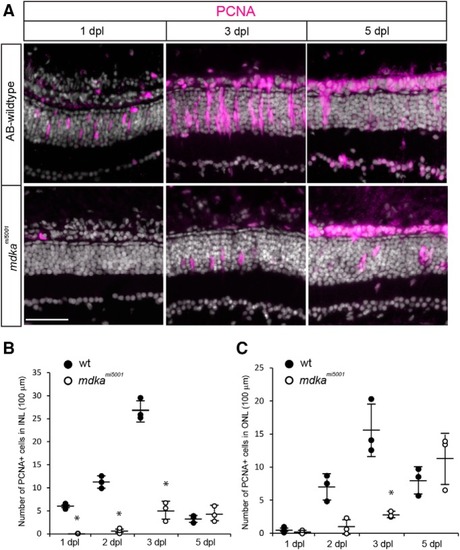
Some Müller glia in the PHENOTYPE:
|
|
The PHENOTYPE:
|
|
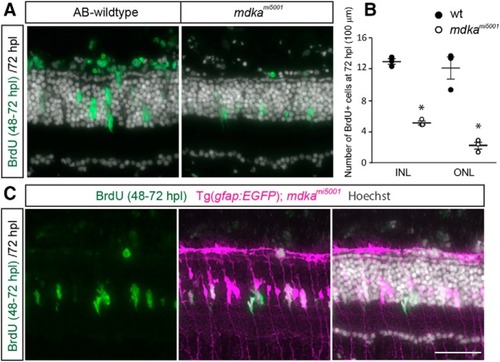
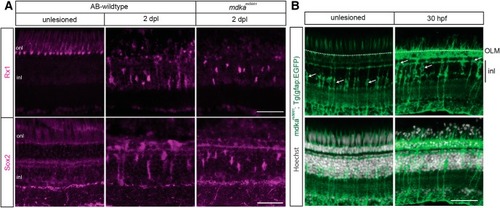
Following photoreceptor death, Müller glia in the |
|
Müller glia in the EXPRESSION / LABELING:
|
|
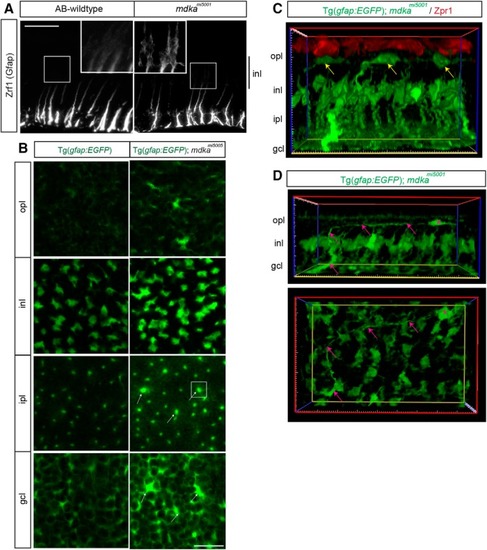
The |
|
Müller glia in the |
|
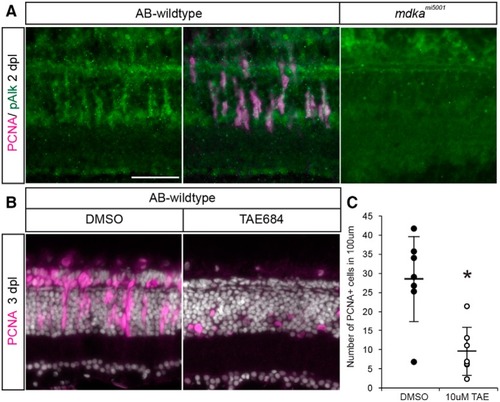
Activation of the ALK receptor is required for Müller glial to proliferate. |
|
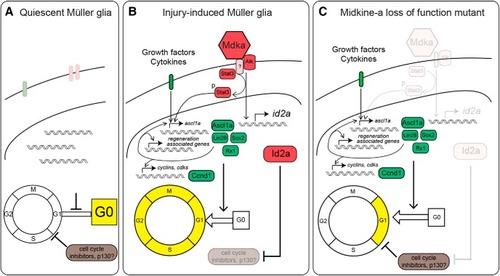
Model of Midkine-a-mediated cell cycle regulation in Müller glia. |