- Title
-
BMP2 Is Related to Hirschsprung's Disease and Required for Enteric Nervous System Development
- Authors
- Huang, S., Wang, Y., Luo, L., Li, X., Jin, X., Li, S., Yu, X., Yang, M., Guo, Z.
- Source
- Full text @ Front. Cell. Neurosci.
|
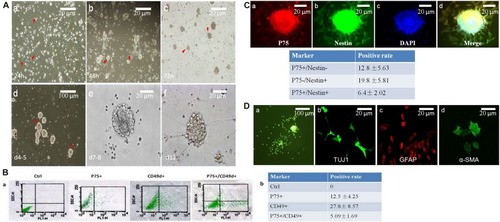
Low or no expression of BMP2 and GDNF in the spasm segment of Hirschsprung disease. |
|
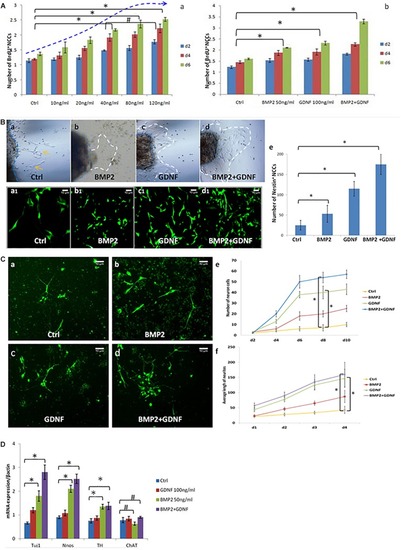
Neurospheres maintain the charactors for self-renewable and multipotent NCCs. |
|
BMP2 promotes the proliferation migration and differentiation of NCCs. |
|
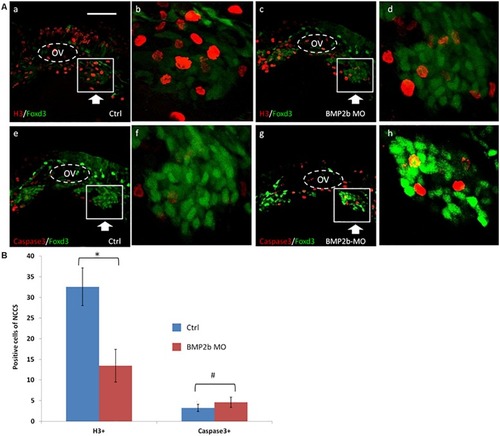
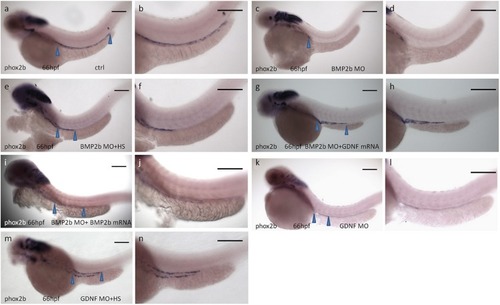
BMP2b is required for normal ENS and intestinal smooth muscle development. PHENOTYPE:
|
|
Proliferation but not apoptosis was changed in vagal NCC and ENS NCC in BMP2b morphants. Here we selected the areas included in the squares to count the positive cells, and the square areas were magnified on the right side of each image. PHENOTYPE:
|
|
BMP2 function are required for differentiation of neurons. PHENOTYPE:
|
|
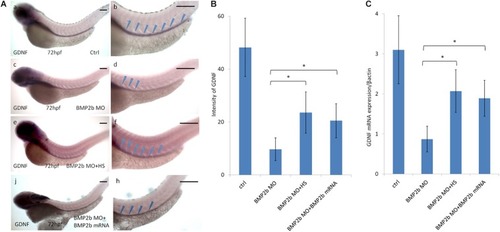
Expression of GDNF in the intestinal mesenchyme requires BMP2 signaling. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Rescue of BMP2-MO and GDNF-MO phenotypes by heat shock induced BMP2 using Tg(Hsp70:bmp2b-GFP) embryos, as well as by injecting BMP2b mRNA or GDNF mRNA. |