- Title
-
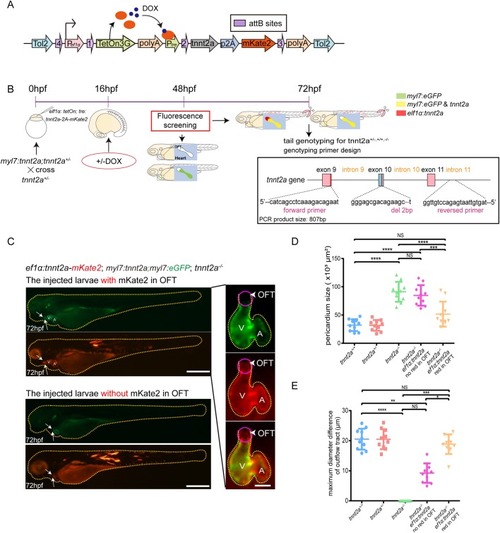
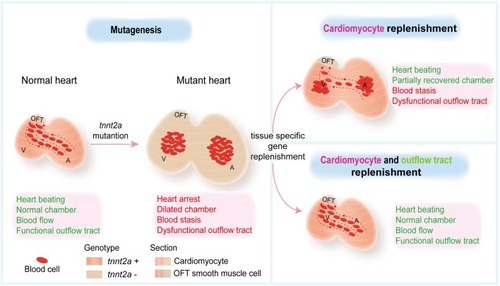
Combinatorial genetic replenishments in myocardial and outflow tract tissues restore heart function in tnnt2 mutant zebrafish
- Authors
- Liu, L., Fei, F., Zhang, R., Wu, F., Yang, Q., Wang, F., Sun, S., Zhao, H., Li, Q., Wang, L., Wang, Y., Gui, Y., Wang, X.
- Source
- Full text @ Biol. Open
|
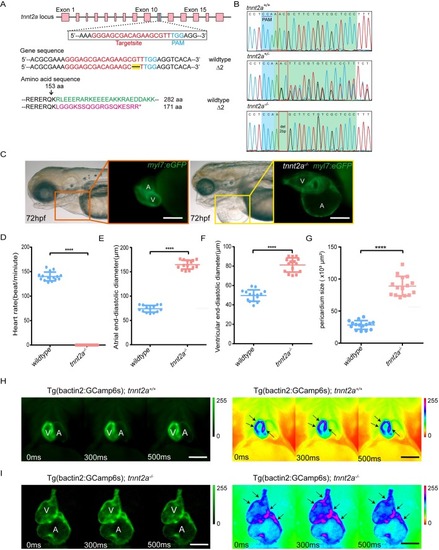
EXPRESSION / LABELING:
PHENOTYPE:
|
|
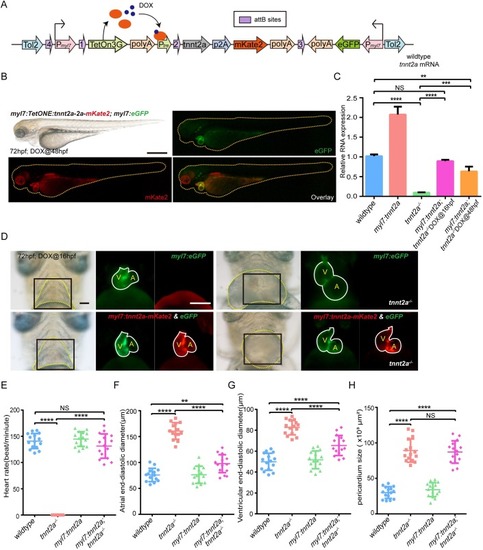
EXPRESSION / LABELING:
PHENOTYPE:
|
|
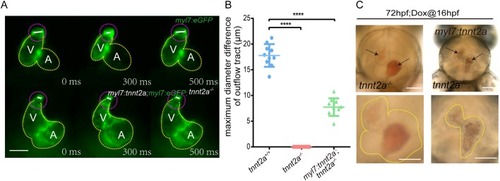
EXPRESSION / LABELING:
PHENOTYPE:
|
|
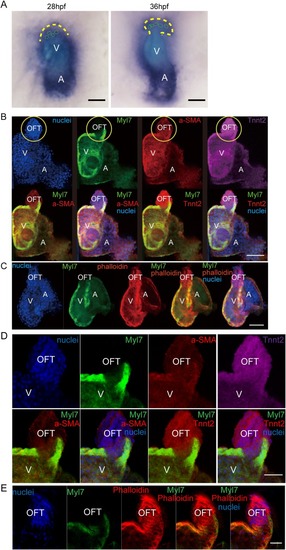
EXPRESSION / LABELING:
PHENOTYPE:
|
|
|
|
|
|
|