- Title
-
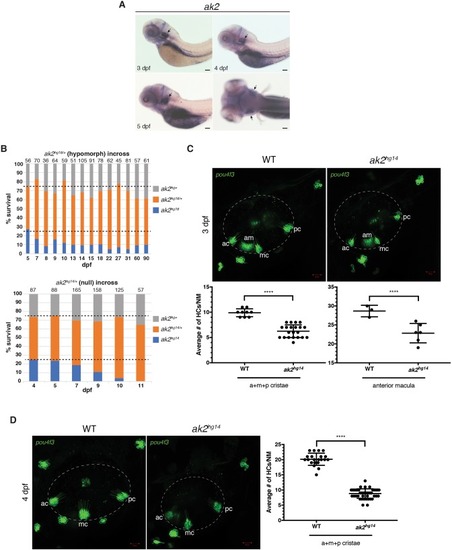
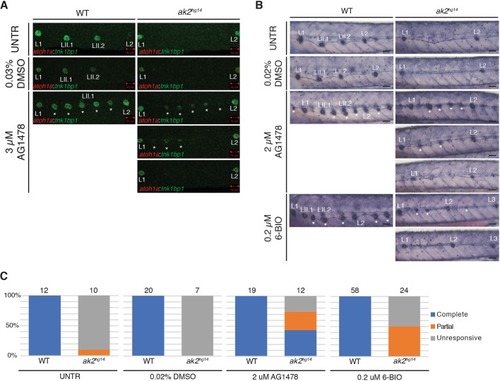
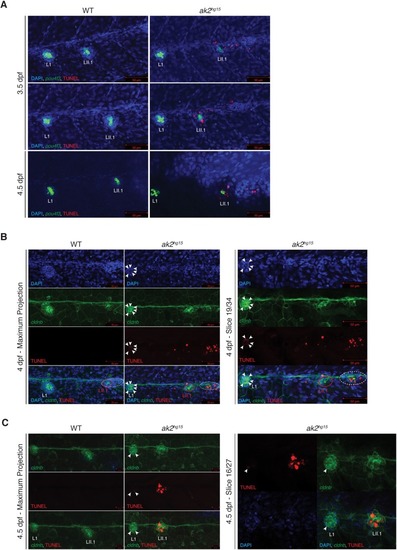
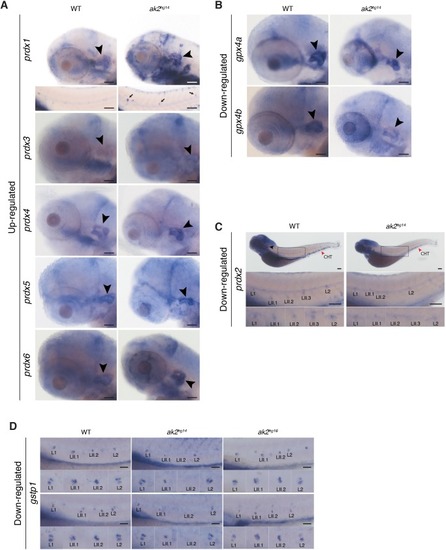
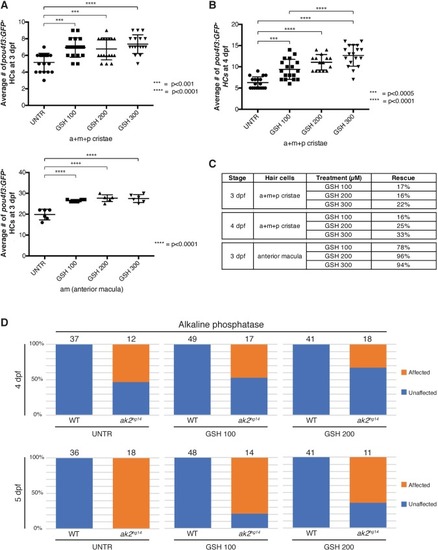
A model for reticular dysgenesis shows impaired sensory organ development and hair cell regeneration linked to cellular stress
- Authors
- Rissone, A., Jimenez, E., Bishop, K., Carrington, B., Slevin, C., Wincovitch, S.M., Sood, R., Candotti, F., Burgess, S.M.
- Source
- Full text @ Dis. Model. Mech.
|
EXPRESSION / LABELING:
PHENOTYPE:
|
|
PHENOTYPE:
|
|
PHENOTYPE:
|
|
EXPRESSION / LABELING:
PHENOTYPE:
|
|
PHENOTYPE:
|
|
PHENOTYPE:
|
|
|
|
PHENOTYPE:
|