- Title
-
Pyridoxamine Supplementation Effectively Reverses the Abnormal Phenotypes of Zebrafish Larvae With PNPO Deficiency
- Authors
- Chen, P.Y., Tu, H.C., Schirch, V., Safo, M.K., Fu, T.F.
- Source
- Full text @ Front Pharmacol
|
Schematic diagram of vitamin B6 metabolism. B6 vitamers, including pyridoxine (PN), pyridoxal (PL), and pyridoxamine (PM), can be phosphorylated to pyridoxine 5′-phosphate (PNP), pyridoxal 5′-phosphate (PLP), and pyridoxamine 5′-phosphate (PMP) by pyridoxal kinase. The phosphorylated form of B6 vitamers can also be hydrolyzed by pyridoxal phosphatase. The conversion of PNP/PMP to PLP requires an active pyridoxine 5′-phosphate oxidase (PNPO). |
|
Structural and phylogenic comparison of zebrafish Pnpo with enzymes from four different sources. The amino acid sequences of PNPOs from the indicated species were analyzed and compared for functional domains and evolutionary conservation. |
|
Spatial and temporal distribution of zebrafish |
|
The impact of knocking down zPnpo. EXPRESSION / LABELING:
PHENOTYPE:
|
|
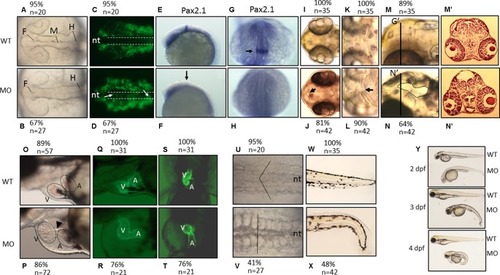
The morphological characteristics of zPnpo morphants. Zebrafish embryos of wild-type and transgenic lines injected with EXPRESSION / LABELING:
PHENOTYPE:
|
|
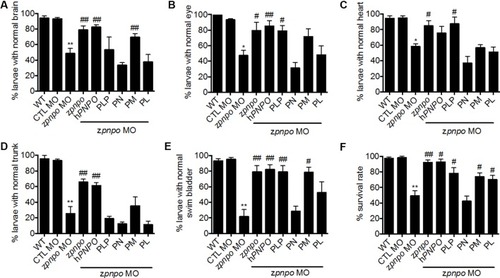
Rescue of zPnpo morphant morphology. Embryos injected with 5 ng of zPnpo MO at one- to two-cell stage were co-injected with 800 pg of z PHENOTYPE:
|
|
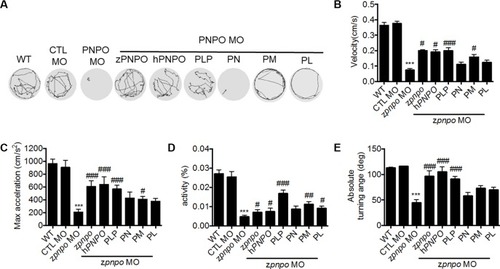
The locomotor activity of zPnpo morphants at 24 hpf. Embryos injected with 5 ng of zPnpo MO at one- to two-cell stages were co-injected with 800 pg of z PHENOTYPE:
|
|
The locomotor activity of zPnpo morphants at 4 dpf. Zebrafish larvae with the indicated treatment were placed individually in each well on a 48-well plate and rested in the tested chamber for 5 min before recording. PHENOTYPE:
|

Unillustrated author statements EXPRESSION / LABELING:
PHENOTYPE:
|