- Title
-
NAD+ improves neuromuscular development in a zebrafish model of FKRP-associated dystroglycanopathy
- Authors
- Bailey, E.C., Alrowaished, S.S., Kilroy, E.A., Crooks, E.S., Drinkert, D.M., Karunasiri, C.M., Belanger, J.J., Khalil, A., Kelley, J.B., Henry, C.A.
- Source
- Full text @ Skelet Muscle
|
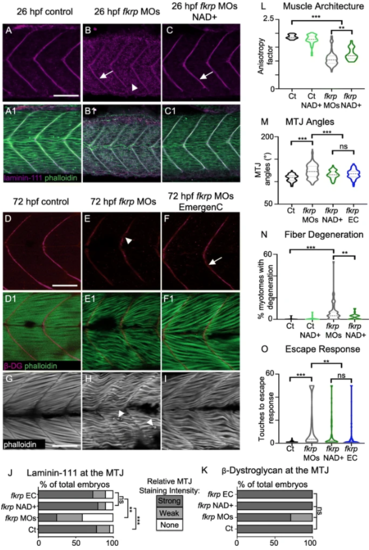
NAD+ or EmergenC supplementation at gastrulation improves muscle structure and function in fkrp morphants. (A–C) Anterior left, dorsal top, side-mounted embryos at 26 hpf stained for laminin-111 (purple) and actin (phalloidin, green). (A, A1) Control embryo. Laminin is concentrated at the MTJ. (B, B1) fkrp morphant. Although laminin is present at the MTJ (white arrow), it is also present within the myotome (white arrowhead). (C, C1) fkrp morphant treated with 0.1 mM NAD+ at 6 hpf. Laminin is concentrated at the MTJ (white arrow) as in control embryos. (D–F1) Anterior left, dorsal top, side-mounted embryos at 72 hpf stained for beta-DG (red) and f-actin (phalloidin, green). (D, D1) Control embryo. (E, E1) fkrp morphant. Beta-DG staining appears weaker at the MTJ (white arrowhead). (F, F1) fkrp morphant treated with EmergenC at 6 hpf. Beta-DG staining appears stronger at the MTJ (white arrow). (G–I) Anterior left, dorsal top, side-mounted embryos at 72 hpf stained for f-actin (phalloidin, gray). (G) Control embryo. (H) fkrp morphant. White arrowheads indicate single detached fibers. (I) fkrp morphant treated with EmergenC at 6 hpf. Note the improved muscle fiber structure. (J) Relative staining intensity of laminin-111 at the MTJ in 26 hpf embryos, based on no staining/localization (none, white), weak staining/localization (weak, light gray), and strong staining/localization (strong, dark gray) (images were blinded prior to analysis, see the “Methods” section). Controls (n = 23 embryos) have greater laminin intensity staining than fkrp morphants (n = 32 embryos). NAD+ (n = 10 embryos) and EmergenC (n = 30 embryos) supplementation improve laminin-111 concentration at the MTJ in fkrpmorphants. (K) Relative staining intensity of beta-DG at the MTJ in 72 hpf embryos, based on weak staining (weak, light gray) and strong staining (strong, dark gray). Although fkrp morphants have more embryos with weaker beta-DG staining at the MTJ (n = 7 embryos), there is no significant difference in beta-DG at the MTJ between untreated morphants, morphants treated with NAD+ (n = 7 embryos), EmergenC (n = 7 embryos), or control morphants (n = 5 embryos). (L) Quantification of muscle organization at 72 hpf. The anisotropy factor in embryos injected with fkrpMOs (n = 96 half-myotomes) is reduced compared to uninjected controls (n = 16 half-myotomes), and NAD+ (n = 40 half-myotomes) significantly increases the anisotropy factor in fkrp morphants. (M) Quantification of MTJ angles at 72 hpf. Injection of fkrp MOs (n = 343 MTJs) significantly increases MTJ angles compared to uninjected controls (n = 98 MTJs). Either NAD+ (n = 105 MTJs) or EmergenC (n = 158 MTJs) treatment significantly reduces MTJ angles compared to untreated morphants. (N) Untreated fkrp morphants (n = 54 embryos) have a significantly higher percent of myotomes per embryo with fiber detachments than controls (n = 40 embryos) at 72 hpf. NAD+ (n = 31 embryos) supplementation significantly reduces the percent of myotomes with dystrophy per embryo. (O) Injection of fkrpMOs (n = 184 embryos) significantly increases the number of touches required to induce an escape response compared to uninjected controls (n = 185 embryos). NAD+ (n = 84 embryos) or EmergenC (n = 111 embryos) treatment significantly reduces the number of touches to invoke an escape response. Scalebars are 50 μm. *p < 0.05, **p < 0.01, ***p < 0.001, ns non-significant PHENOTYPE:
|
|
NAD+ and EmergenC supplementation at gastrulation do not significantly improve the UPR or vascularization in fkrp morphants. (A–C1) Anterior left, dorsal top, side-mounted 3 dpf embryos expressing Tg (ef1α:xbp1δ-GFP). Fluorescence intensity was kept constant within an experiment (see methods). Numbered panels are merged with the brightfield channel. (A, A1) Control embryo. Note the low relative expression of Xbp1 compared to morphants. (B, B1) fkrp morphant. (C, C1) fkrp morphant treated with EmergenC at gastrulation. Note that fluorescence intensity is similar to that of untreated morphant. (D) Quantification of Xbp1 fluorescence intensity as a percent of the wild-type value for all groups imaged. Fluorescence intensity is significantly increased in morphants. There is no significant difference in fluorescence intensity between untreated morphants (n = 15 embryos) and morphants receiving EmergenC (n = 21 embryos). (E–G) Anterior left, dorsal top, side-mounted 2 dpf embryos expressing Tg (fli1:EGFP) focused on the ISVs. (E) Control embryo. (F) fkrp morphant embryo. Note that some ISVs are truncated (white arrowhead). (G) fkrp morphant embryo treated with NAD+ at gastrulation. Truncated ISVs are still present in NAD+ treated morphants (white arrowhead). (H) Quantification of ISV length. ISV length is reduced in fkrp morphants (n = 201 vessels) compared with uninjected controls (n = 84 vessels). NAD+ supplementation (n = 166 vessels) does not rescue ISV length in fkrp morphants. Scalebars are 50 μm. *p < 0.05, **p < 0.01, ***p < 0.001, ns non-significant PHENOTYPE:
|
|
Paxillin overexpression does not improve muscle structure in fkrp morphants. (A–B3) Anterior left, dorsal top, side-mounted embryos at 26 hpf stained for Paxillin (green) and f-actin (purple). Paxillin concentrates at the MTJ (white arrowhead) in control embryos. There are some gaps in Paxillin localization in fkrp morphants (B2, white arrow) and Paxillin accumulates at the muscle pioneers (B2, white asterisk). (C–E2) Anterior left, dorsal top, side-mounted embryos at 72 hpf stained for f-actin (phalloidin, gray) in lettered and even numbered panels and expressing Paxillin-EGFP (green) in numbered panels. (C–C2) A fkrp morphant with disorganized muscle fibers. (D–D2) Control Tg (hsp70l:pxna-EGFP) embryo expressing Paxillin which concentrates at the MTJ (white arrowhead). (E–E2) Tg (hsp70l:pxna-EGFP) fkrp morphant. (F) MTJ angle quantification. Fkrp morphants with (n = 241 MTJs) and without (n = 170 MTJs) Paxillin-EGFP expression have a significant increase in average MTJ angle width compared with uninjected controls (n = 189, 191 MTJs). Paxillin overexpression in fkrp morphants significantly increases MTJ angles compared to morphants that do not overexpress Paxillin. (G) Fiber degeneration quantification. Fkrp morphants with (n = 55 embryos) and without (n = 60 embryos) Paxillin-EGFP expression have a significant increase in the percent of myotomes with degeneration per embryo compared to uninjected controls (n = 57, 54 embryos). Although there is a trend, Paxillin-overexpressing morphants do not have a significant reduction in dystrophy compared to control fkrp morphants. (H) Escape response quantification. Fkrpmorphants with (n = 88 embryos) and without (n = 70 embryos) Paxillin-EGFP expression require significantly more touches to induce an escape response compared with uninjected controls (n = 66, 80 embryos). Fkrp morphants overexpressing Paxillin do not exhibit a significant improvement in escape response compared with fkrp morphants that do not overexpress Paxillin. Scalebars are 50 μm. *p < 0.05, **p < 0.01, ***p < 0.001, ns non-significant PHENOTYPE:
|
|
Later NAD+ supplementation improves MTJ structure, but NAD+ and EmergenC are required prior to initial muscle development to improve motility, fiber resilience, and fiber organization. (a–d) Anterior left, dorsal top, side-mounted embryos at 72 hpf stained for f-actin (phalloidin, gray). (a) Control embryo. (b) fkrp morphant. (c) fkrp morphant treated with NAD+ at 24 hpf. (d) fkrp morphant treated with EmergenC at 24 hpf. White arrowheads indicate single fiber detachments. (e) Fiber organization quantification. The anisotropy factor in embryos injected with fkrp MOs (n = 104 half-myotomes) is reduced compared to controls (n = 48 half-myotomes) and not improved with NAD+ supplementation at 24 hpf (n = 96 half-myotomes). (f) MTJ angle quantification. Fkrpmorphants treated with NAD+ (n = 381 MTJs) or EmergenC (n = 318 MTJs) at 24 hpf have significantly decreased MTJ angles compared to untreated morphants (n = 651 MTJs). (g) Fiber detachment quantification. Although there is a trend towards reduced muscle degeneration, fkrp morphants receiving NAD+ (n = 55 embryos) or EmergenC (n = 41 embryos) at 24 hpf do not have a significant reduction in the percent of myotomes with dystrophy compared to untreated morphants (n = 98 embryos). (h) Escape response quantification. The number of touches required to induce an escape response is elevated in embryos injected with fkrp MOs (n = 128 embryos) compared to controls (n = 114 embryos). Morphants treated with NAD+ at 24 hpf (n = 81 embryos) have a worsened escape response compared to untreated morphants. Morphants treated with EmergenC at 24 hpf (n = 52 embryos) do not exhibit a significant change in escape response compared to untreated morphants, but significantly differ from NAD+-treated morphants. Scalebars are 50 μm. *p < 0.05, **p < 0.01, ***p < 0.001, ns non-significant PHENOTYPE:
|
|
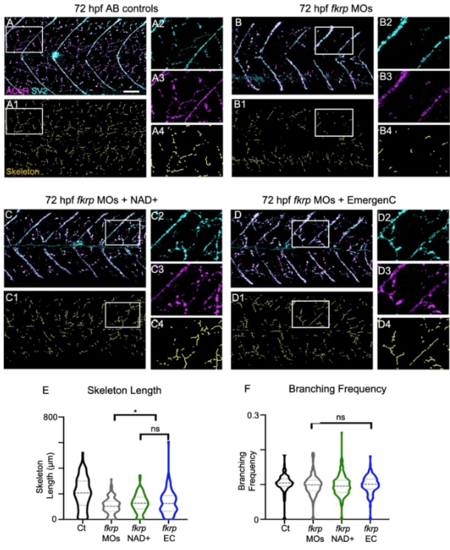
Fkrp morphants exhibit NMJ defects and NAD+ and EmergenC treatment prior to muscle development improves NMJ development. (A–D4) Anterior left, dorsal top, side-mounted embryos at 72 hpf with labeled AChR and SV2. (Lettered panels) Merged channels of AChR and SV2. (1) Skeletonized images. (2) Magnification of SV2 channel. (3) Magnification of AChR channel. (4) Magnification of skeleton channel. (A–A4) Control embryo. (B–B4) fkrpmorphant embryo exhibiting reduced distributed innervation within the myotome. (C–C4) fkrp morphant embryo treated with NAD+ at 6 hpf shows increased NMJs. (D–D4) fkrpmorphant embryo treated with EmergenC at 6 hpf also shows increased NMJs. (E) Length of skeletons. Skeleton length is reduced in fkrp morphants (n = 153 half-myotomes) compared to controls (n = 144 half-myotomes) but significantly increased in fkrpmorphants receiving NAD+ (n = 183 half-myotomes) or EmergenC (n = 127 half-myotomes) at 6 hpf. (F) Degree of branching within the myotome in control embryos (n = 141 half-myotomes), fkrp morphants (n = 147 half-myotomes), and fkrp morphants receiving NAD+ (n = 179 half-myotomes) or EmergenC (n = 126 half-myotomes) at 6 hpf. Scalebars are 50 μm. *p < 0.05, **p < 0.01, ***p < 0.001, ns non-significant PHENOTYPE:
|
|
Skeleton length is less disrupted in dag1 morphants than in fkrp morphants and is not significantly improved with NAD+ supplementation. (A–C6) Anterior left, dorsal top, side-mounted embryos at 72 hpf with labeled actin, AChRs, and SV2. (Lettered panels) Phalloidin stained embryos. (1) Merged channels of AChR and SV2. (2) Skeletonized images. (3) Magnification of phalloidin channel. (4) Magnification of SV2 channel. (5) Magnification of AChR channel. (6) Magnification of skeleton channel. (A–A6) Control embryo. (B–B6) dag1 morphant embryo. (C–C6) dag1 morphant embryo treated with NAD+ at 6 hpf. (D) Length of skeletons per myotome in control embryos (n = 186 half-myotomes), dag1 morphants (n = 260 half-myotomes), and dag1 morphants receiving NAD+ (n = 252 half-myotomes) at 6 hpf. Skeleton length is not significantly different between untreated and NAD+ treated dag1 morphants. (E) Degree of branching within the myotome in control embryos (n = 180 half-myotomes), dag1 morphants (n = 254 half-myotomes), and dag1 morphants receiving NAD+ (n = 249 half-myotomes) at 6 hpf. NAD+ treatment made no significant difference. (F) MTJ angle quantification. MTJ angles are significantly reduced in dag1 morphants receiving NAD+ (n = 98 MTJs) compared to untreated dag1morphants (n = 97 MTJs). (G) Bar graph of the percent skeleton length of control embryos per myotome. Note that the average percent skeleton length is more drastically reduced in fkrp morphants (51.4%, n = 153 half-myotomes) than in dag1 morphants (83.9%, n = 260 half-myotomes). NAD+ supplementation increased this percentage in fkrp morphants (61.9%, n = 183 half-myotomes) but has little effect on dag1 morphants (85.0%, n = 252 half-myotomes) Scalebars are 50 μm, error bars in (G) are standard error of the mean. *p < 0.05, **p < 0.01, ***p < 0.001, ns non-significant PHENOTYPE:
|
|
Supplementation with NAD+ or EmergenC after muscle development does not improve NMJ morphology in fkrp morphants. (A-D4) Anterior left, dorsal top, side-mounted embryos at 72 hpf with labeled AChRs and SV2. (Lettered panels) Merged channels of AChR and SV2. (1) Skeletonized images. (2) Magnification of SV2 channel. (3) Magnification of AChR channel. (4) Magnification of skeleton channel. (A–A4) Control embryo. (B–B4) fkrp morphant embryo exhibiting a reduced degree of distributed innervation within the myotome. (C–C4) fkrp morphant embryo treated with NAD+ at 24 hpf also has reduced innervation. (D–D4) fkrp morphant embryo treated with EmergenC at 24 hpf has reduced NMJs. (E) Length of skeletons per myotome in control embryos (n = 288 half-myotomes), fkrp morphants (n = 303 half-myotomes), and fkrp morphants receiving NAD+ (n = 225 half-myotomes) or EmergenC (n = 225 half-myotomes) at 24 hpf. Note that skeleton length is actually significantly decreased in fkrp morphants receiving NAD+ at 24 hpf. (F) Degree of branching within the myotome in control embryos (n = 283 half-myotomes), fkrpmorphants (n = 295 half-myotomes), and fkrp morphants receiving NAD+ (n = 221 half-myotomes) or EmergenC (n = 210 half-myotomes) at 24 hpf. Scalebars are 50 μm. *p < 0.05, **p < 0.01, ***p < 0.001, ns non-significant PHENOTYPE:
|
|
Muscle-specific overexpression of Fkrp improves MTJ angles, but not motility or fiber resiliency. (A–B2) Anterior left, dorsal top, side-mounted embryos at 72 hpf stained for f-actin (phalloidin, gray) and expressing Fkrp-EGFP (green). (A–A2) Control Tg (hsp70l:fkrp-EGFP) embryo expressing Fkrp in the fibers. (B–B2) fkrp morphant Tg (hsp70l:fkrp-EGFP) embryo expressing Fkrp in fibers. (C) MTJ angles of Tg (hsp70l:fkrp-EGFP) control (n = 264 MTJs) and fkrp morphant (n = 340 MTJs) embryos and AB controls and morphants (n = 141, 194 MTJs) at 72 hpf. Constitutive expression of Fkrp improves MTJ angles in morphants. (D) Constitutive expression of Fkrp in morphants (n = 72 embryos) significantly reduces the number of myotomes with fiber degeneration compared with control morphants (n = 35 embryos). (E) The escape response is significantly reduced in fkrp morphants constitutively overexpressing Fkrp (n = 82 embryos) compared to control morphants (n = 44 embryos). (F–H2) Anterior left, dorsal top, side-mounted embryos at 72 hpf stained for f-actin (phalloidin, gray) and expressing Fkrp-EGFP under control of the muscle-specific -503unc promoter (green). (F) Control Tg(-503unc:fkrp-EGFP) embryo expressing Fkrp specifically in muscle fibers. G) fkrp morphant embryo on Tg(-503unc:fkrp-EGFP) background lacking Fkrp expression in muscle fibers. (H) fkrp morphant Tg(-503unc:fkrp-EGFP) embryo expressing Fkrp specifically in muscle fibers does not show improved muscle organization. (I) Muscle specific expression of FKRP in fkrp morphants (n = 158 MTJs) significantly improves MTJ angles compared to control morphants (n = 153 MTJs). (J) Muscle-specific overexpression of Fkrp (n = 45 embryos) does not significantly lower the percent of myotomes with muscle degeneration in control fkrp morphants (n = 47 embryos). (K) There is not a significant difference in the number of touches required to induce an escape response in fkrp morphants that overexpress Fkrp (n = 73 embryos) in muscle versus those that do not (n = 79 embryos). Scalebars are 50 μm. *p < 0.05, **p < 0.01, ns non-significant PHENOTYPE:
|