- Title
-
Neddylation Facilitates the Antiviral Response in Zebrafish
- Authors
- Yu, G., Liu, X., Tang, J., Xu, C., Ouyang, G., Xiao, W.
- Source
- Full text @ Front Immunol

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. |
|
Zebrafish larvae treated with MLN4924 are more sensitive to SVCV infection. (A)Representative images of zebrafish larvae (3 dpf), both uninfected and infected with SVCV for 24 h, after treatment with the vehicle (DMSO; the control) or MLN4924 (1 μM). Dead larvae (indicated with red arrows) were characterized by a lack of movement, absence of blood circulation, and bodily degeneration. (B) Survival ratios indicated that zebrafish larvae treated with MLN4924 were more sensitive to SVCV infection than were larvae treated with vehicle (DMSO). Zebrafish larvae (3 dpf; n = 90 in total) were infected with SVCV (2 × 108 TCID50/ml) after pretreatment with either vehicle (DMSO) or MLN4924 (1 μM); this experiment was repeated three times (n = 30 for each). We counted the numbers of dead larvae at 8, 16, 24, 32, 40, and 48 h post-infection. (C–E) Viral replication was much greater in SVCV-infected zebrafish larvae treated with MLN4924 (1 μM), as compared with the control. Zebrafish larvae were infected with SVCV after pretreatment with either vehicle (DMSO; the control) or MLN4924 (1 μM). After incubation for 24 h, we used qRT-PCR assays to determine the expression levels of the SVCV genes P (C), G (D), and N genes (E). Data are shown as mean ± SEM of three independent experiments, each performed in triplicate; the statistical analysis was performed using GraphPad Prism 5 (unpaired t-test).
|
|
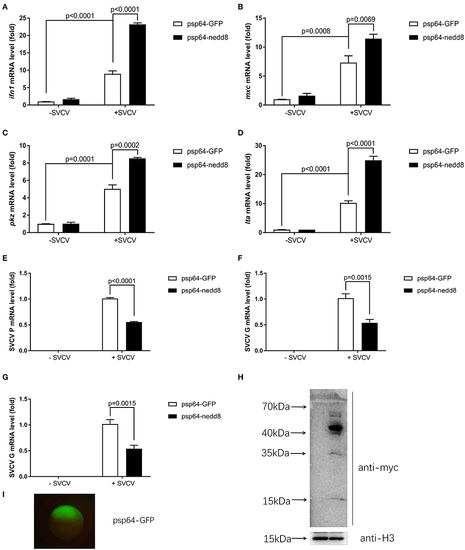
Overexpression of nedd8 upregulates key antiviral genes after SVCV infection and suppresses viral replication in vivo. (A–D) Ectopic expression of nedd8, induced by mRNA injection upregulated key antiviral genes in SVCV infected zebrafish larvae. We injected zebrafish embryos at the one-cell stage with either GFP mRNA (400 pg/per embryo) or Myc-tagged nedd8 mRNA (400 pg/per embryo). At 3 dpf, we added SVCV viruses (2 × 108 TCID50/ml) into the water containing zebrafish larvae. After incubation for 24 h, we extracted total RNA from all larvae and performed qPCR assays to detect the expression levels of ifn1 (A), mxc (B), and pkz (C) and lta (D). (E–G) Ectopic expression of nedd8 by mRNA injection suppressed SVCV replication in embryos. We performed qRT-PCR assays to detect the expression levels of the SVCV genes P (E), G (F), and N (G) genes of SVCV. (H,I) Western blot assay and Fluorescence micrographs of zebrafish embryos showed the expression levels of injected GFP mRNA or Myc-nedd8 mRNA. Data are shown as mean ± SEM of three independent experiments, each performed in triplicate; the statistical analysis was performed using GraphPad Prism 5 (unpaired t-test).
|

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. |
|
nedd8-null adult zebrafish are more sensitive to SVCV infection than WT zebrafish. (A,B) nedd8-null zebrafish (3 mpf; 0.38 ± 0.02 g) and the WT (3 mpf; 0.38 ± 0.02 g) were each i.p. injected with 10 μL SVCV (~2 × 108 TCID50/ml) at 0 day. (C,D) At 1 day post-injection (dpi), there were no obvious differences between the WT (nedd8+/+) and nedd8-null zebrafish (nedd8−/−). (E,F) At 2 dpi, the WT zebrafish appeared normal, but the nedd8-null zebrafish had more swelling and hemorrhagic symptoms in the abdomen (indicated by red arrows).
PHENOTYPE:
|

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. |

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. |