- Title
-
The Glycogen Synthase Kinase-3β Inhibitor LSN 2105786 Promotes Zebrafish Fin Regeneration
- Authors
- Sarmah, S., Curtis, C., Mahin, J., Farrell, M., Engler, T.A., Sanchez-Felix, M.V., Sato, M., Ma, Y.L., Chu, S., Marrs, J.A.
- Source
- Full text @ Biomedicines
|
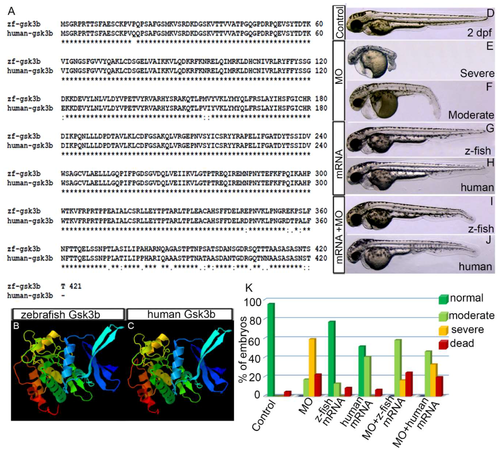
Zebrafish Gsk3β is structurally and functionally equivalent to human GSK3Β. (A) Alignment of protein sequences of zebrafish and human gsk3β. Identical residue (*), conserved (:), and semi conserved (.) substitutions are marked. (B,C) 3D-protein structure of zebrafish (B) and human (C) gsk3β. (D–J) Live image of the 2 dpf control embryo (D). (E,F) Gsk3β translation blocking morpholino oligonucleotide (MO) injection (3.5 ng/µL) produced severe and moderate defects. Live images of the severely (E) and moderately (F) defective morphant embryos. (G,H) Live images of the embryos injected with zebrafish Gsk3β and human GSK3β mRNA. (I,J) Live images of the embryos co-injected with either MO or zebrafish Gsk3β mRNA or MO and human GSK3β mRNA. (K) Graph showing the percentage of phenotypes of the embryos in different conditions: MO alone, zebrafish Gsk3β mRNA alone, human GSK3β mRNA, MO + zebrafish Gsk3β mRNA, MO + human GSK3β mRNA. |
|
LSN 2105786 upregulates Wnt Signaling and produces eyeless phenotype. (A–I) Embryos treated with LSN 2105786 displayed the absent or small eye phenotype in a dose dependent manner characteristic of over-activated Wnt signaling at 28 hpf. (J) Live image of DMSO treated embryo showing normal eye at 28 hpf. (K–S) Embryos treated with LSN 2105786 displayed the absent or small eye phenotype in a dose dependent manner characteristic of over-activated Wnt signaling at 48 hpf. Panels E’, F’, G’, N’ are second examples of each concentration. (T) Live image of DMSO treated embryo showing normal eye at 48 hpf. (U–X) Bright field (U,W) and green fluorescence (V,X) images of the midbrain-hindbrain boundary region of Tg(TOP:GFP) embryos treated with DMSO (U,V) or 450 nM LSN 2105786 (W,X) at 24 hpf. (Y) Graph showing the GFP expressing midbrain-hindbrain boundary region of the embryos treated with different concentrations of LSN 2105786. Compared to DMSO, increased GFP volume in 300–450 nM was statistically significant (p < 0.001), and 500 nM effect was not statistically significant. |
|
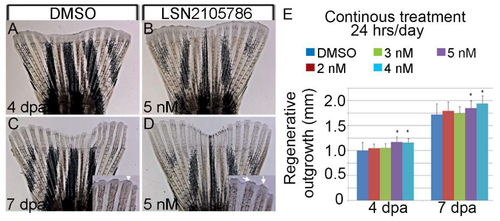
LSN 2105786 treatment accelerates fin regeneration. (A–D) Fin rays showing regeneration after DMSO (A,C) and LSN 2105786 treatment (B,D) at 4 (A,B) and 7 dpa (C,D). Insets show the mature fin tips in the LSN 2105786 treated fins (arrows) compared to DMSO treated fins (arrow head). (E) Graphs shows that continuous treatment of 4 and 5 nM LSN 2105786 produces increased regenerate lengths at 4 and 7 dpa compared to DMSO treatment. *, p < 0.05. |
|
LSN 2105786 treatment increased expression of Wnt targets and downstream genes in regenerating fin tissue. (A–F) In situ hybridization detecting lef1 transcript showed that the expression was restricted to the area in (arrowhead) and immediately surrounding (arrow) the blastema in DMSO treated fins, whereas, 5 nM LSN 2105786 treated fins showed an expansion of the expression domain as well as intensified staining at 1 and 2 dpa (A–D). Staining intensity at 3 dpa was not significantly altered in 5 nM LSN 2105786 treated fins compared to DMSO treated fins, but the expression area was expanded distally in treated fins (E,F). (O–R) In situ hybridization detecting shh transcript showed the expression in two distinct subsets of cells at the distal tip of the DMSO treated fins at 3 and 7 dpa (O,Q). Fins treated continuously with 5 nM LSN 2105786 showed expansion of the expression domain (P,R). (G–J) The expression of bmp2b was similar in the DMSO treated and 5 nM LSN 2105786 treated fins at 3 and 4 dpa. (K–N) In situ hybridization detecting bmp4transcript showed that the expression was dramatically increased in the 5 nM LSN 2105786 treated fin at 3 dpa (L) compared to DMSO treated fin (K). At 4 dpa, bmp4 was robustly expressed in the distal most epidermis of LSN 2105786 treated fin (N) but was not detected in the same region of DMSO treated fins (M). Scale bars in panels P and R are 3 cm. Bar in P applies for all panels, except Q and R. Bar in R applies to Q and R. |
|
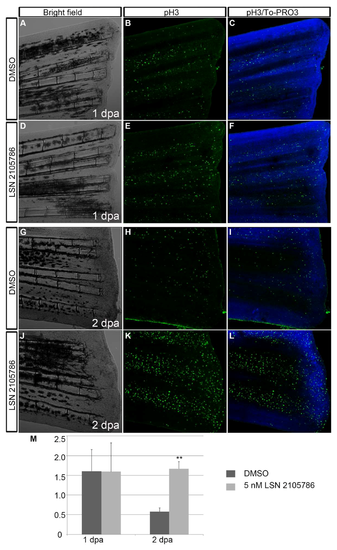
LSN 2105786 treatment increases cell proliferation in regenerating fins. (A–L) Phosphohistone 3 immunostaining showed similar labeling of proliferated pH3 positive cells in the DMSO treated (B,C) and 5 nM LSN 2105786 treated fins at 1 dpa (E,F), but at 2 dpa, 5 nM LSN 2105786 treated fins (K,L) showed dramatic increase of pH3 positive cells compared to DMSO treated fins (H,I). (M) Graph shows the number of pH3 positive cells at 1 and 2 dpa. **, p < 0.001. |
|
LSN 2105786 treatment increases osteoblast differentiation. (A–F) Zns-5 antibody staining showed an expansion of the labeling area in the blastema region in 5 nM LSN 2105786 treated fins (E,F) compared to DMSO treated fins (B,C) at 2 dpa. Yellow boxed areas are shown in high magnification. (G–L) Zns-5 antibody staining of 7 dpa fins showed Zns-5 labeling in the lateral regions of the hemirays of DMSO treated fish (H,I), but 5 nM LSN 2105786 treated fish exhibited Zns-5 positive cells in the lateral regions of the hemirays and in the joints between the segments (K,L). Arrows point to the joints. (M–R) High magnification images of the boxed areas are shown in G–L. The 5 nM LSN 2105786 treated fins show Zns-5 labeling in the distal region of the bony rays (N,O) compared to DMSO treated fins (Q,R). |