- Title
-
Midbrain tectal stem cells display diverse regenerative capacities in zebrafish
- Authors
- Lindsey, B.W., Aitken, G.E., Tang, J.K., Khabooshan, M., Douek, A.M., Vandestadt, C., Kaslin, J.
- Source
- Full text @ Sci. Rep.
|
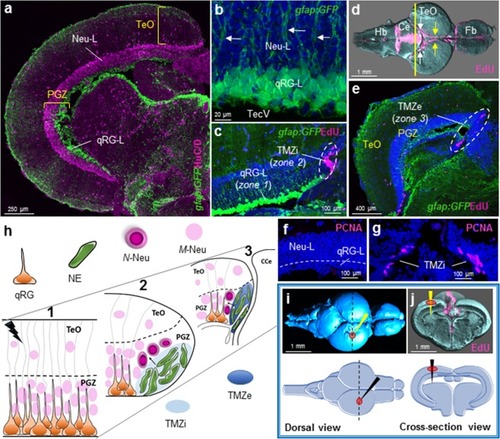
Tectal composition, stem cell niches, and stab lesion model. |
|
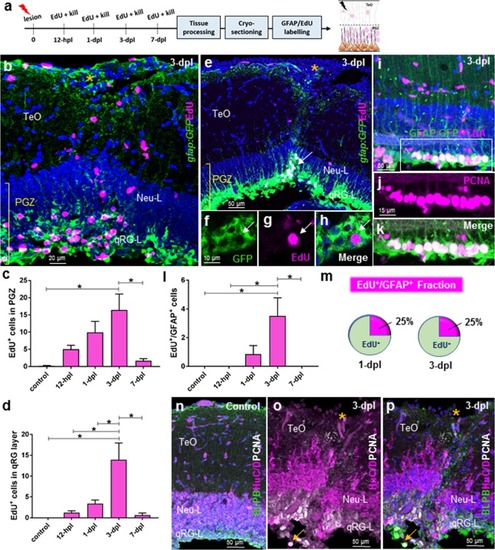
Histological response following tectal lesion. |
|
Cell proliferation post-lesion in the qRG layer of the periventricular grey zone (PGZ; stem cell zone 1). |
|
Cell proliferation post-lesion in the TMZi (stem cell zone 2) and TMZe (stem cell zone 3). |
|
Differentiation post-lesion in activated qRG of the qRG-layer. |
|
Differentiation post-lesion in NE amplifying progenitors in the TMZi. |
|
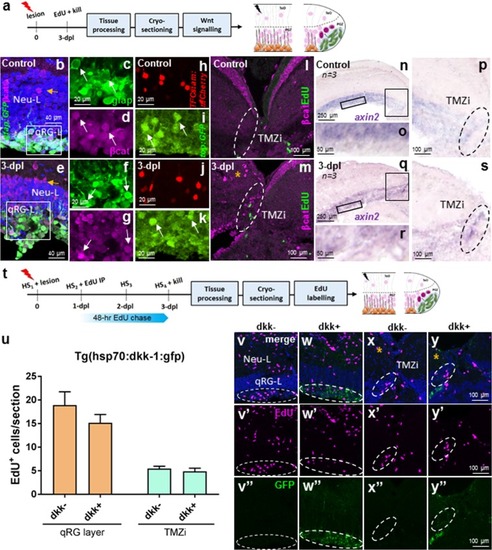
Wnt/β-catenin signalling in stem cell zone 1 (qRG-L) and zone 2 (TMZi) following tectal lesion. |