- Title
-
Six6 and Six7 coordinately regulate expression of middle-wavelength opsins in zebrafish
- Authors
- Ogawa, Y., Shiraki, T., Asano, Y., Muto, A., Kawakami, K., Suzuki, Y., Kojima, D., Fukada, Y.
- Source
- Full text @ Proc. Natl. Acad. Sci. USA
|
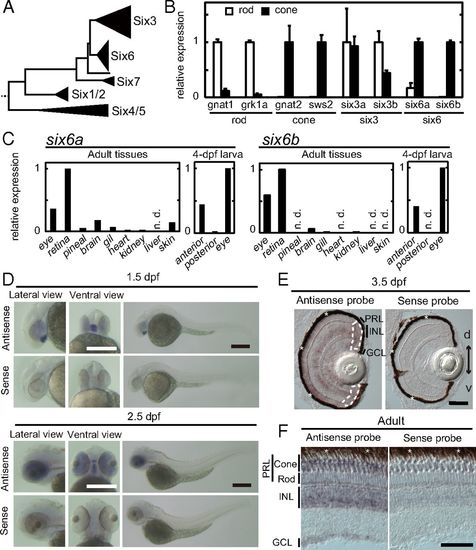
Expression pattern of six6a and six6b. (A) Schematic representation of the phylogenetic tree of the Six family, modified from our previous study (17). Relative expression levels of six6a and six6b are shown in isolated rods and cones at the adult stage (mean ± SD, n = 2) (B) and in adult and 4-dpf larval tissues (C). n.d., not detected. Photoreceptor purity after isolation was further validated in SI Appendix, Fig. S1. Expression patterns of six6b examined by in situ hybridization in whole-mount embryos at 1.5 dpf and 2.5 dpf (D), in the larval retina at 3.5 dpf (E), and in the 6-mo postfertilization adult retina (F) are shown. In E, ciliary marginal zones are surrounded by white broken lines. d, dorsal side; GCL, ganglion cell layer; INL, inner nuclear layer; PRL, photoreceptor layer; v, ventral side. The retinal pigmented epithelium (indicated by asterisks) is adjacent to the photoreceptor layer. (Scale bars: D, 300 μm; E and F, 50 μm.) |
|
Compensatory regulation of sws2 and rh2 opsin genes by six6b and six7. (A) Schematic drawing of the transgene construct. (B) EGFP expression in the anterior region of six6b-tg (ja70Tg) at 5 dpf. (Scale bar: 200 μm.) (C) EGFP signals detected in retinal photoreceptor layers of 4-dpf six6b-tg larvae (green). Weak EGFP signals were also detected in the inner nuclear layer (also SI Appendix, Fig. S2A), consistent with previous work (12). RH2 and LWS cones were labeled with zpr1 antibody (magenta). Cell nuclei were counterstained with DAPI (blue). (Scale bar: 40 μm.) (D and E) Relative mRNA levels of opsin genes and six6b in the 5-dpf larval eyes [mean ± SEM, n = 4 independent samples analyzed for each genotype; *P < 0.05, Tukey’s multiple comparison test (D), Student’s t test (E)]. n.s., not significant. (F) Expression pattern of cone opsin genes examined by in situ hybridization using the 5-dpf larval eyes. Magnified view of the photoreceptor layer (box surrounded with white lines) is indicated on the left side of each panel. The retinal pigmented epithelium is indicated by asterisks. d, dorsal side; v, ventral side. (Scale bar: 50 μm.) (G) Related to F. Quantification of sws2- or rh2-1/2–positive photoreceptors in the central retina (mean ± SEM; *P < 0.05, Tukey’s multiple comparison test). The numbers of fish used for quantification are indicated in the bar graph. |
|
Compromised development of cone photoreceptors in six6a/six6b/six7 TKO zebrafish. (A) Exon/intron organization and partial nucleotide sequences of zebrafish six6a and six6b. The binding sites of the left and right TAL effector nucleases are highlighted in blue. The recognition sites of the restriction endonucleases PvuII and XhoI are highlighted in green. The nucleotide sequences of the KO fish alleles (ja62 for six6a, ja63 and ja64 for six6b) are compared with the WT sequence. Deletions are indicated by dashes. Six6a and Six6b and their mutant proteins are represented as schematic drawings. The frameshift position is indicated by an arrowhead. (B) Genotyping of the six6a and six6b mutants by PCR. The asterisk indicates 500 bp. Expression profiles of rod and cone opsin genes in the larval eyes at 4 dpf (C; mean ± SEM, n = 4 for each genotype; *P < 0.05, Student’s t test) and at 5 dpf (D; mean ± SEM, n = 5 for each genotype; *P < 0.05, Tukey’s multiple comparison test) are shown. n.s., not significant. The n value refers to the number of independent samples analyzed. (E) Expression pattern of cone opsin genes examined by in situ hybridization using 5-dpf larval eyes. A magnified view of the photoreceptor layer (dotted box) is indicated on the left side of each panel. The retinal pigmented epithelium is indicated by asterisks. d, dorsal side; v, ventral side. (Scale bar: 50 μm.) (F) Fluorescent images of the flat-mounted retinas prepared from the adult WT and TKO. The retinas were immunostained with zpr1 antibody, which recognizes RH2 and LWS cones (green) and with DRAQ5 to highlight cell nuclei (magenta). (Scale bar: 20 μm.) (G, Left) Fluorescent images in retinal cryosections from the adult fish labeled for TUNEL (red). The cell nuclei were counterstained with DAPI (blue). TUNEL-positive cells are indicated by arrowheads. (Scale bars: 30 μm.) (G, Right) Quantification of TUNEL-positive cells in the central or peripheral retina. The numbers of TUNEL-positive cells were counted for each cryosection and averaged (mean ± SEM, n = 55 for WT, n = 38 for TKO; *P < 0.05, Student’s t test vs. WT). n.s., not significant. |
|
Impaired prey capture in six6a/six6b/six7 TKO zebrafish at 6 dpf. (A) Time-lapse images during a typical hunting episode. Paramecia are indicated by orange arrowheads. (Scale bar: 100 μm.) (B) Relative numbers of paramecia counted in the chamber containing WT and TKO larvae. The number of the paramecia left in the chamber was normalized to the initial count. The data are represented by mean ± SD (n = 12 for each genotype). (C) Relative numbers of paramecium at 10 min (mean ± SEM, n = 12; *P < 0.05, Student’s t test). (D) Distribution of eye vergence angle. The data are represented by mean ± SD (n = 12). (E) Frequency of eye convergence during 10 min. The eye convergence was defined as the state in which the eye vergence angle was more than 70°. A vergence angle greater than 70° is indicated by the bar in D. (F) Swimming speed of WT and TKO larvae. Values in E and F are presented as mean ± SEM (n = 12; *P < 0.05, Student’s t test). PHENOTYPE:
|

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. EXPRESSION / LABELING:
|

Unillustrated author statements |