- Title
-
3-ketodihydrosphingosine reductase mutation induces steatosis and hepatic injury in zebrafish
- Authors
- Park, K.H., Ye, Z.W., Zhang, J., Hammad, S.M., Townsend, D.M., Rockey, D.C., Kim, S.H.
- Source
- Full text @ Sci. Rep.
|
Progression of liver injury in the KdsrI105R zebrafish mutant. Whole mount oil-red O (ORO) staining in wild type control sibling (A, left) and kdsr mutant (B, left) at 6 days post fertilization (dpf) and 7 dpf. ORO staining performed in transverse section of the liver (L, right panels of A and B). N = 10 per each. Lipid accumulation in the heart (h) and blood vessel (bv) are marked in images of panel B. H & E staining results in control at 7 dpf (left), mutant at 7 dpf (middle), and mutant at 8 dpf (right) (C, n = 6 per each). The magnified area depicts hepatocyte ballooning in mutants at 7 dpf. Images shown are representative of at least 10 other zebrafish or livers, respectively. Scale bars = 100 µm (whole mount ORO staining in A and B), 40 µm (ORO staining on the liver section in A and B), and 100 µm (H&E staining in C). The predicted protein structure of kdsr and the locus of the mutated amino acid is shown in (D). Relative mRNA expression of tnfa, il1b, and col1a1a in the wild type and mutant siblings is shown in (E). *P ≤ 0.05. |
|
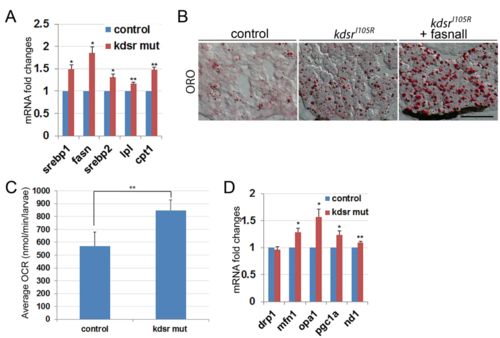
Lipid metabolism- and mitochondrial homeostasis-associated gene expression, and oxygen consumption analysis in larvae. Relative mRNA expression of genes associated with lipid metabolism include sterol regulatory element-binding protein 1 (srebp1), fatty acid synthase (fasn), srebp2, lipoprotein lipase (lpl), and carnitine-palmitoyltransferase I (cpt1) ( A). Oil red O staining in control, kdsrI105R mutant, and kdsrI105R mutant siblings after fasnall treatment at 7 dpf; 1 µM fasnall treatment was performed from 4 dpf to 7 dpf ( B). Images shown are representative of at least 10 in total. Scale bar in B = 100um. Average oxygen consumption rate was measured from 7 groups (3 larvae per group) of control and mutant siblings for 30 minutes ( C). Relative mRNA expression of genes associated with mitochondrial homeostasis include dynamin related protein 1 (drp1), mitofusin 1 (mfn1), optic atrophy type 1 (opa1), peroxisome proliferator-activated receptor gamma coactivator 1-alpha (pgc1a), and NADH-ubiquinone oxidoreductase chain1 (nd1) ( D). Error bars indicate standard deviation of the mean. *P ≤ 0.05, **P ≤ 0.005. |
|
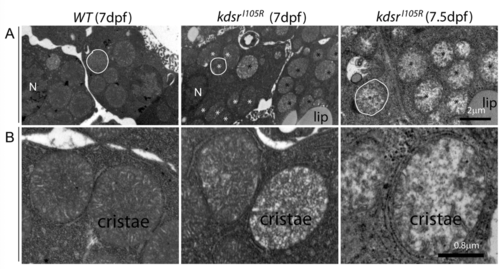
Transmission electron microscopy (TEM) imaging of hepatocytes. 8,000x magnification in wild type at 7 dpf (left), kdsrI105R mutant at 7 dpf with mild phenotype (middle), and kdsrI105R mutant at 7.5 dpf with severe defect (right) ( A). One of representative mitochondria is outlined with white color. N, nucleus. Normal looking mitochondria and abnormal mitochondria were marked with white and black asterisks, respectively. 20,000x magnification images are shown in ( B). Images shown are representative of 2 other animals. Scale bars = 2 µm (panel A), 0.8 µm (panel B). Lip, lipid drops. PHENOTYPE:
|

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. |
|
Sphk2 deletion suppresses liver defects in the kdsrI105R mutant. Images of live wild type (wt), kdsrI105R, and kdsrI105R; sphk2wc1 double mutant at 7 dpf (A). Scale bar = 0.25 mm. Relative mRNA expression of sphk2 and other genes associated with inflammation (tnfa, il1b), tissue injury (alpha1-anti-trypsin (a1at)) and fibrosis (col1a1a) (B), sphingolipid salvage pathway related genes (C) in wt, kdsrI105R, sphk2wc1, and kdsrI105R; sphk2wc1 mutant siblings. spp1, (S1P-phosphohydrolase 1). Images of H&E and ORO staining in livers of wt, kdsrI105R and kdsrI105R; sphk2wc1double mutants (D, n = 6). Error bars indicate standard deviation of the mean. *P ≤ 0.05, **P ≤ 0.005. Scale bare in D = 100 µm. |

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. |

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. |

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. |
|
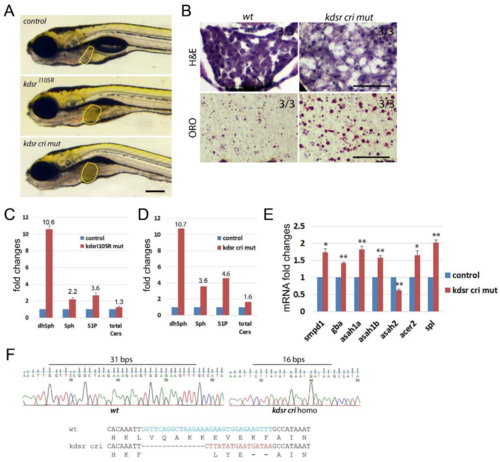
Supporting Fig. 3. The kdsrcri mutant generated by CRISPR gene targeting and sphingolipids profiling. Wild type and the kdsrcri mutant images at 7 dpf (A), Scale bar=0.25 mm. H & E staining and ORO staining in control (B, left), mutant at 7 dpf (B, right). Scale bar=50µm. Relative amount of sphingolipids from 30 control siblings and 30 kdsrI105R mutant larvae siblings at 7 dpf (C) and 30 control siblings and 30 kdsrcri mutant larvae siblings at 7 dpf (D). Relative mRNA expression in sphingolipid salvage pathway components in control siblings and kdsrcri mutants at 7 dpf (E). CRISPR/Cas9 RNA injection generated premature stop codon in exon3 of kdsr in the kdsrcri mutant by 31 bps deletion and 16 bps insertion (F). * P≤0.05, ** P≤0.005. |
|
kdsrI105R/cri biallelic mutants showed hepatomegaly and darker liver at 8 dpf. Liver is outlined with yellow line. Scale bar=0.2mm. |