- Title
-
Loss-of-function of sox3 causes follicle development retardation and reduces fecundity in zebrafish
- Authors
- Hong, Q., Li, C., Ying, R., Lin, H., Li, J., Zhao, Y., Cheng, H., Zhou, R.
- Source
- Full text @ Protein Cell

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. |
|
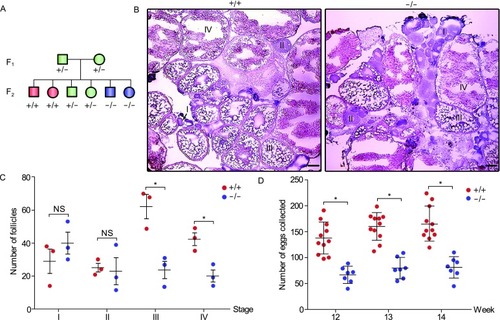
Knockout of sox3 leads to follicle development retardation and a reduced fecundity in female zebrafish. (A) Schematic diagram of heterozygote incrosses. All data are from the progeny derived from heterozygote incrosses. WT, +/+; heterozygotes, +/−; homozygotes, −/−. (B) Histological analysis of adult ovaries of sox3+/+ and sox3−/− by H.E. staining. I, primary growth stage; II, cortical alveolus stage; III, early-vitellogenic stage; IV, late-vitellogenic stage. Scale bar, 50 μm. (C) Statistic analysis of different stages of follicles from 10 sections for each ovary of sox3+/+and sox3−/− (n = 3 fish per genotype). Data represent means ± SEM. T-test was performed. *P < 0.05; **P < 0.01. NS, not significant. (D) Impaired fecundity in sox3−/−. Statistical analysis of the numbers of eggs produced by sox3−/− incrosses or sox3+/+ incrosses at 12-, 13- and 14-week. Data represent means ± SEM. T-test was performed. *P < 0.05
PHENOTYPE:
|
|
Up-regulated apoptosis in the sox3−/− ovaries in comparison with wild type ovaries. (A) Statistical analysis of the apoptosis-related DEGs (FDR < 0.05) in both sox3+/+ and sox3−/−based on KEGG analysis. Red bars indicated pro-apoptotic genes and blue bars represented anti-apoptotic genes. The genes with fold change of 1.4 were listed. (B) Hierarchical clustering indicated the expression levels of apoptosis-related DEGs between sox3+/+ and sox3−/− ovaries. (C) Quantitative real-time PCR analysis of selected genes to validate the DEGs from RNA-seq data. The transcript levels were related to β-actin expression. Relative level, 2−ΔΔCt. T-test was performed. *P< 0.05; **P < 0.01. (D) Western blot analysis showed that cleaved-caspase3 were up-regulated in the sox3−/− ovaries in comparison with the wild type ovaries. Gapdh was used as an internal control. (E) TUNEL analysis. In follicles of stages III and IV, obvious signals (FITC-labeled, green) were observed in somatic cells (theca cells and granulosa cells) (white arrowheads) of sox3−/− ovaries. The nuclei were stained by PI (red). The enlarged images originated from the white squares. The ovaries sections were stained by H.E. and showed on the right. Scale bar: 100 µm
|
|
Disrupted pathway of 17β-E2 synthesis and up-regulated apoptosis in the sox3−/−ovaries. (A) The expression levels of cyp19a1a in both sox3+/+ and sox3−/− ovaries based on RNA-seq data. (B) Quantitative real-time PCR analysis of cyp19a1a expression levels in both sox3+/+ and sox3−/− ovaries. The transcript levels were related to β-actin expression. Relative level, 2−ΔΔCt. T-test was performed. *P < 0.05. (C) The standard curve of 17β-E2 concentration at OD450. (D) Down-regulated 17β-E2 in sox3−/− gonads in comparison with the wild type gonads. Data represented means ± SEM. T-test was performed. *P < 0.05; **P < 0.01. (E) Immunofluorescence analysis of Sox3 protein in adult ovary. Anti-Sox3 and FITC-conjugated goat anti-rabbit IgG (H + L) antibodies were used to detect Sox3 (green). The nuclei were stained by Hoechst (blue). Preimmune serum was used as a negative control. The white square area in the inset was enlarged and showed on the right. Sox3 positive signals were observed in somatic cells (theca cells and granulosa cells) (white arrowheads) in ovary. Scale bar, 50 μm. (F) Immunofluorescence analysis of Cyp19a1a protein in adult ovary. Anti-Cyp19a1a and FITC-conjugated goat anti-rabbit IgG (H + L) antibodies were used to detect Cyp19a1a (green). The nuclei were stained by Hoechst (blue). Preimmune serum was used as a negative control. The white square area in the inset was enlarged and showed on the right. Cyp19a1a positive signals were observed in somatic cells (theca cells and granulosa cells) (white arrowheads) in ovary. Scale bar, 50 μm. (G) Assessment of apoptosis using Annexin V-FITC/PI and flow cytometry. CHO cells were treated with 17β-E2 (500 ng/mL) or DMEM (control) for 36 h, and then treated with etoposide (3 μg/mL) for 12 h and assayed by flow cytometry. Viable cells exhibited Annexin V-/PI- (symbol 3 in the plot); early apoptotic cells exhibited Annexin V+/PI-(symbol 4 in the plot); late apoptotic cells exhibited Annexin V+/PI+ (symbol 1 in the plot); necrotic cells and some late apoptotic cells exhibited Annexin V-/PI+ (symbol 2 in the plot). (H) Percentages of both early and late apoptotic cells based on the apoptosis assessment by flow cytometry and Annexin V/PI in (G). T-test was performed. *P < 0.05
|