- Title
-
Syntaxin 1B Mediates Berberine's Roles in Epilepsy-Like Behavior in a Pentylenetetrazole-Induced Seizure Zebrafish Model
- Authors
- Zheng, Y.M., Chen, B., Jiang, J.D., Zhang, J.P.
- Source
- Full text @ Front. Mol. Neurosci.
|
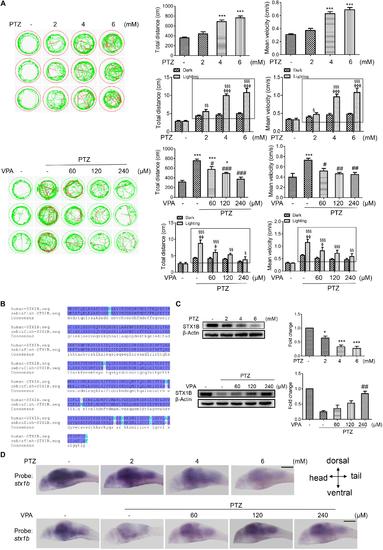
Stx1b gene expression was suppressed in PTZ-induced seizure zebrafish larvae. (A) Seizure-like swimming was induced by PTZ in zebrafish larvae. The left track panel and the right upper histograms show the larval swimming behavior (distance and speed) during 20 min in the dark condition. The red trajectory indicates overactive movement and the green trajectory indicates active movement in the left track figure. The right upper histograms show the larval swimming distance and speed, which were recorded for 20 min in the dark condition. The right lower histograms show the larval swimming distance and speed in three cycles of 5 min dark and 10 s light periods; the open boxes show the differences in distance and velocity of the overexcited larvae between the dark and light conditions (n = 24). (B) Alignment of human and zebrafish STX1B amino acid sequences. The amino acids shown in dark blue are identical, those in shallow blue demonstrate amino acids with similar polarity, and those in white/shallow blue are different. (C) Western blotting tests indicated that STX1B protein was decreased by PTZ (Upper) and increased by VPA (Lower) in a concentration-dependent manner (n = 3). ∗P < 0.05 and ∗∗∗P < 0.001 vs. wild-type; #P < 0.05, ##P < 0.01, and ###P < 0.001 vs. PTZ model; § P < 0.05, §§ P < 0.01, and §§§P < 0.001 vs. wild-type in the light condition; ΦP < 0.01, ΦΦP < 0.01 and ΦΦΦP < 0.001 indicated light vs. dark in the same set of conditions. (D) Hybridization in situ results show STX1B gene expression in the larval brain inhibited by PTZ and rescued by VPA in a concentration-dependent manner (n = 20). EXPRESSION / LABELING:
PHENOTYPE:
|
|
Larval seizure was aggravated by downregulation of stx1b transcription in the PTZ-induced seizure model. Levels of stx1b gene transcription (n = 20) (A) and STX1B protein (n = 3) (B) were reduced and c-fos gene transcription was increased in the larval (7 dpf) brain (n = 20) (C) by stx1b morpholino oligos injection in the PTZ model, as compared with in the PTZ-only and the morpholino oligos-only injection models. The larval swimming experiment (n = 24) (D) showed that average speed and total distance were not changed, but that the abnormal pathway and overspeed were increased following 20 min in the dark condition and that photosensitive seizure was aggravated under the condition of light–dark transition with 5 min in the dark and 10 s in the light for three cycles in the PTZ plus stx1b morpholino oligos larvae, as compared with the two groups of the PTZ-only and the stx1b morpholino oligos-only injection models. The data show average speeds during the 20 min in the dark and the 10 s in the dark–light transformation; the boxes indicate the difference of locomotion distances and speeds between the light–dark transitions. Swimming tracks were recorded at 2 min in the dark condition and the red trajectory indicates overactive movement and the green trajectory indicates active movement. stx1b-MO1 and stx1b-MO2 were two morpholino oligos that bound to the stx1b messenger RNA initiate sequence with a different sequence; by using two target oligos, their inhibition effect was confirmed with each other. ∗∗∗P < 0.001 vs. wild-type; #P < 0.05 and ###P < 0.001 vs. PTZ model; §§P < 0.01 and §§§P < 0.001 vs. wild-type in the light condition; ΦΦP < 0.01 and ΦΦΦP < 0.001 indicates light vs. dark. |
|
Larval seizure-like behavior was reduced by increased STX1B level in a PTZ-induced seizure model. (A) Both stx1b transcription in the wild-type and PTZ models were enhanced in larval (7 dpf) brains by stx1b injection as compared with that following no injection and mock injection (n = 20). (B) Western blotting confirmed differential levels of STX1B protein among the variant groups; notably, the STX1B level was raised in the stx1b-PTZ group as compared with in the PTZ-only group (n = 3). (C) c-fos messenger RNA was decreased by STX1B overexpression in the stx1b-PTZ model versus in the PTZ model group or the mock-PTZ group (n = 20). (D) The larval swimming experiment showed that, with STX1B overexpression, average speed and total distance were not obviously changed but abnormal pathway and overspeed were significantly decreased with 20 min in the dark condition, while photosensitive seizure was inhibited under the condition of light–dark shift in the PTZ-model larvae as compared with in the two groups of the PTZ-only model and the PTZ plus mock injection model (n = 24). The rectangles indicate differential responses between light–dark transitions in the three groups of the PTZ-model larvae. ∗∗P < 0.01 and ∗∗∗P < 0.001 vs. wild-type; #P < 0.05 and ##P < 0.01 vs. PTZ model; §§ P < 0.01 and §§§ P < 0.001 vs. wild-type in the light condition; ΦΦΦP < 0.001 indicates light vs. dark. |
|
Berberine (BBR) inhibited seizure in PTZ-model zebrafish with the increase of STX1B level. (A) A behavioral experiment showed that BBR inhibited the larval overexcited locomotion in speed and distance under the conditions of non-stimulation and dark–light cycling stimulation in PTZ-model larvae. A representative swimming trajectory (2 min) is presented (n = 24). The rectangles showed differential distances and speeds between light–dark transitions in the groups of PTZ plus BBR larvae as compared with in the PTZ-only model. (B) Hybridization in situ showed that BBR inhibited the increase of c-fos and rescued stx1b descending induced by PTZ in the larval (7 dpf) brains (n = 20). (C) Western blotting results confirmed that BBR recovered STX1B protein levels to almost normal in a dose-dependent manner (n = 3). ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001 vs. wild-type; #P < 0.05, ##P < 0.01, and ###P < 0.001 vs. PTZ model; § P < 0.05, §§ P < 0.01, and §§§ P < 0.001 vs. wild-type in the light condition; ΦP < 0.01 and ΦΦP < 0.01 indicates light vs. dark. |
|
Downregulation of STX1B weakened the effects of BBR on anticonvulsant in the PTZ-induced seizure zebrafish model. (A) Hybridization in situ showed that there was a change in the c-fos messenger RNA level in the larval (7 dpf) brain that was induced by BBR in the PTZ plus stx1b morpholino oligos group versus in the three control groups of wild-type, stx1b morpholino oligos injection, and PTZ plus stx1b morpholino oligos (n = 20). (B,C) STX1B downregulation attenuated the efficiency of BBR inhibition on larval overexcited locomotion in terms of speed and distance under non-stimulation conditions and eliminated the action of BBR under dark–light transitions. Swimming trajectories are presented in 2 min recording charts; red tracks indicate over locomotion, while the rectangles indicate the difference between light–dark transitions (n = 24). &P < 0.05, &&P < 0.01, and &&&P < 0.001 vs. PTZ plus stx1b morpholino oligos model; 𝜃P < 0.05, 𝜃𝜃P < 0.01, and 𝜃𝜃𝜃P < 0.001 vs. stx1b morpholino oligos model; §P < 0.05 and §§§P < 0.001 vs. wild-type in the light condition; ΦP < 0.01 and ΦΦP < 0.01 indicates light vs. dark. |
|
Stx1b morpholino oligos injection suppressed BBR activation on STX1B expression in the PTZ-model larvae. (A) Hybridization in situ results show that a change of the stx1b messenger RNA level in the larval (7 dpf) brain was induced by BBR with stx1b morpholino oligos injection in the PTZ-model zebrafish, as compared with in the wild-type, stx1b morpholino oligos injection, and PTZ plus stx1b morpholino oligos groups (n = 20). (B) Western blotting results indicated a change of the STX1B protein level similar to the change of the stx1b messenger RNA level under the same treatments (n = 3). &P < 0.05 vs. PTZ plus stx1b morpholino oligos model; 𝜃P < 0.05 and 𝜃𝜃P < 0.01 vs. stx1b morpholino oligos model. |

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. |