- Title
-
Neuromuscular junction abnormalities in a zebrafish loss-of-function model of TDP-43
- Authors
- Bose, P., Armstrong, G.A.B., Drapeau, P.
- Source
- Full text @ J. Neurophysiol.
|
Significant morphological defects and reduced survival in hom/hom mutants. A: images of 48-h postfertilization larvae of the following different genotypes: wild type (WT), tardbp−/−, tardbpl−/−, het/hom, and hom/hom. Scale bar, 1 mm. Arrow indicates inflated pericardium in het/hom and hom/homlarvae. B–D: body length (B), eye diameter (C), and pericardial area (D) were measured for each treatment group. hom/hom mutants displayed significantly reduced body length and eye diameter and increased pericardial area (**P < 0.01). Additionally, het/hom larvae also displayed a significantly increased pericardial area (*P < 0.05). E: percent survival of WT, tardbp−/−, tardbpl−/−, het/hom, and hom/hommutant larvae tracked for 12 days. All hom/hom mutants died between 4 and 6 days (P < 0.01). Data are means ± SE; N = 2, n = 62. Numbers in parentheses indicate sample sizes.
|
|
Significant locomotor and coiling defects in hom/hom mutants. A and B: burst count per minute (A) and burst duration (B) were measured for the following different genotypes: wild type (WT), tardbp−/−, tardbpl−/−, het/hom, and hom/hom at 17 h postfertilization (hpf). hom/hom mutants show a significantly reduced burst count per minute compared with all the other different genotypes. C: 4 superimposed locomotor path traces of 48-hpf larvae from the following different genotypes: WT, tardbp−/−, tardbpl−/−, het/hom, and hom/hom. D–F: swim duration (D), swim distance (E), and maximum velocity (F) were measured for each treatment group in the touch-evoked escape response (TEER) assay. hom/hom mutants displayed significant locomotor defect in all parameters of the TEER assay compared with all other different genotypes (**P < 0.01). Additionally, het/hom mutant larvae also showed significantly reduced swim distance compared with WT, tardbp−/−, and tardbpl−/− larvae (*P < 0.05). Numbers in parentheses indicate sample sizes for the TEER assay in each treatment group. Data are means ± SE. PHENOTYPE:
|
|
Significantly reduced miniature end-plate current (mEPC) frequency in hom/hom mutants. A–C: the passive membrane properties membrane potential (Vm; A), capacitance (Cm; B), and membrane resistance (Rm; C) of fast-twitch muscle cells at 48 h postfertilization for the following different genotypes: wild type (WT), tardbp−/−, tardbpl−/−, het/hom, and hom/hom. hom/hom mutants displayed a significantly depolarized membrane potential compared with the other different genotypes (P < 0.01). D: recordings of mEPCs, which result from spontaneous release of quanta, were done. hom/hom mutants showed a significantly reduced frequency (**P < 0.01). *P < 0.05. E: representative mEPCs for 5 min for all different genotypes from a 10-min recording. F: mEPC amplitude. G: overlay of representative individual mEPC from all different genotypes. H and I: decay constant (τ; H) and rise time (I) were measured across all different genotypes. No other significant differences were found. Numbers in parentheses indicate sample sizes. Data are means ± SE. |
|
Significantly compromised neuromuscular junction (NMJ) connectivity in hom/hom mutants. A: representative images of one ventral root projection at 2 days postfertilization (dpf) labeled with synaptotagmin 1 (ZNP-1; presynaptic marker), sulforhodamine-conjugated α-bungarotoxin (αBTX; postsynaptic marker), and merged. B: representative images of one ventral root projection at 3 dpf labeled with ZNP-1 (presynaptic marker), sulforhodamine-conjugated αBTX (postsynaptic marker), and merged. Wild-type (WT) larvae demonstrate tight colocalization of both ZNP-1 and αBTX (merged). Scale bar is 25 μm for all images. C: quantification of orphaned ZNP-1 puncta to total ZNP-1 puncta. At 2 dpf, all other genotypes displayed significantly greater orphaned ZNP-1 puncta compared with WT larvae (one-way ANOVA, post hoc Tukey’s test; #P < 0.05). At 3dpf, all other genotypes displayed significantly greater orphaned ZNP-1 puncta compared with WT larvae (one-way ANOVA, post hoc Tukey’s test; $P < 0.05). Paired t-test between the same group at 2 and 3 dpf indicates a significant difference in het/hom and hom/hom mutants (paired t-test; *P < 0.05; **P < 0.01). D: quantification of orphaned αBTX puncta to total αBTX puncta. At 2 dpf, all other genotypes displayed significantly greater orphaned ZNP-1 puncta compared with WT larvae (one-way ANOVA, post hoc Tukey’s test; #P < 0.05). At 3dpf, all other genotypes displayed significantly greater orphaned ZNP-1 puncta compared with WT larvae (one-way ANOVA, post hoc Tukey’s test; $P < 0.05). Paired t-test between the same group at 2 and 3 dpf indicates a significant difference in tardbp−/− and hom/hom mutants (paired t-test; *P < 0.05; **P < 0.01). Data are means ± SE. Numbers in parentheses indicate sample sizes.
EXPRESSION / LABELING:
PHENOTYPE:
|
|
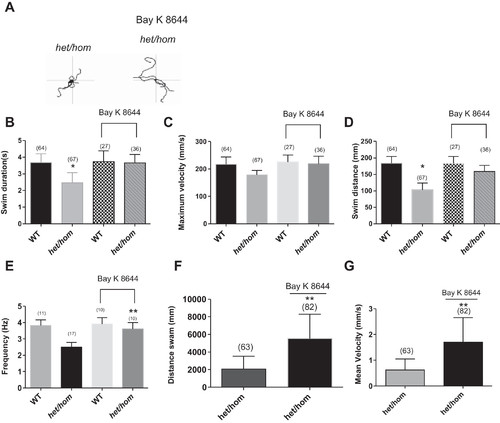
A: 5 superimposed locomotor path traces of 48-h postfertilization (hpf) het/hom mutants without and with chronic application of BAY K 8644 (2 μM). B–D: swim duration (B), swim distance (C), and maximum velocity (D) were measured at 48 hpf in WT and het/hom mutants after chronic application of BAY K 8644 (2 μM) in the touch-evoked escape response (TEER) assay. het/hom mutants with treatment displayed significant recovery from locomotor defect in swim duration and swim distance of the TEER assay compared with het/hom mutants without treatment (paired t-test; P < 0.01). E: recordings of miniature end-plate currents (mEPCs) from 2-day postfertilization (dpf) het/hom mutants after chronic application of BAY K 8644 (2 μM). het/hom mutants showed a significant recovery in mEPC frequency (paired t-test; **P < 0.01). F and G: distance swam (F) and mean velocity (G) at 5 dpf by het/hom mutants untreated and treated with BAY K 8644 (2 μM) for 4 days. **P < 0.01. |