- Title
-
γ-Tubulin small complex formation is essential for early zebrafish embryogenesis
- Authors
- Pouchucq, L., Undurraga, C.A., Fuentes, R., Cornejo, M., Allende, M.L., Monasterio, O.
- Source
- Full text @ Mech. Dev.
|
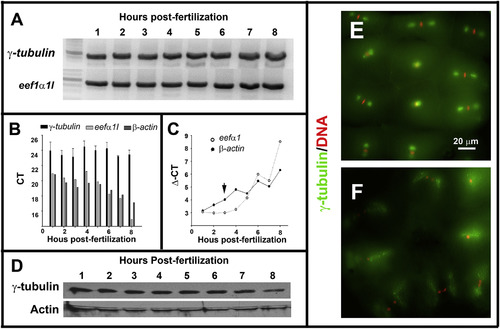
Maternal-zygotic zebrafish γ-tubulin expression and subcellular localization during early development. A. RT-PCR analysis revealed by electrophoresis in agarose gel showed that γ-tubulin mRNA is detected during the first 8 h of the zebrafish embryogenesis. B. qPCR analysis indicated that γ-tubulin mRNA expression levels were invariant during the first 8 h of zebrafish embryogenesis. β-actin and eef1a were used as reference genes. C. Immunoblot analysis showed that γ-tubulin protein is present during the cleavage (1–3 hpf) and post-MBT stages (3–8 hpf) of zebrafish development. Actin was used as a loading control. D–E. Fluorescence microscopy images of zebrafish embryos (2 hpf) double-stained with anti-γ-tubulin antibody (green) and DAPI (red). Metaphase (E), anaphase, and telophase (F) stages of the cell cycle are shown. EXPRESSION / LABELING:
|
|
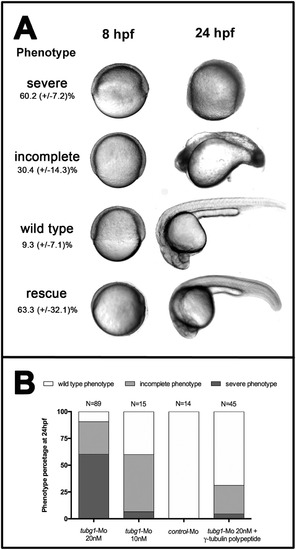
γ-tubulin silencing by morpholino-based knockdown causes developmental arrest during gastrulation. A. Bright field images of the representative phenotypes obtained in a tubg1-Mo (20 nM) injection experiment and complete rescue phenotype. Control injected embryos did not show a phenotype. Images were taken at 8 and 24 hourspost-fertilization (hpf). The frequency of each phenotypic class (severe, moderate and wild type) is indicated as a percentage. The standard deviations correspond to three independent biological replicas. B. Phenotypes distribution of 24 hpf embryos injected with tubg1-Mo (20 and 10 nM), control Mo (20 nM) and tubg1-Mo (20 mM) + γ-tubulin polypeptide. PHENOTYPE:
|

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. EXPRESSION / LABELING:
PHENOTYPE:
|
|
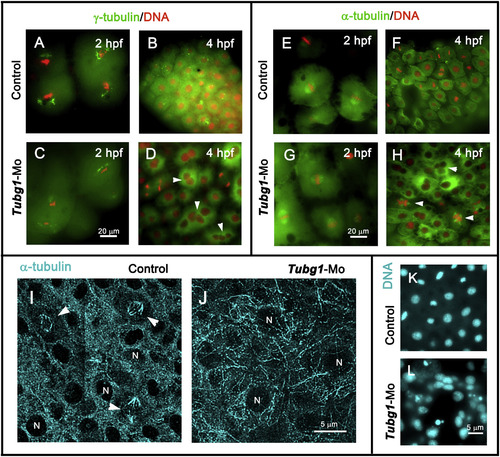
Nuclear and microtubule organization defects in γ-tubulin morphant embryos. A–D. Fluorescence microscopy images of control and morphant embryos at 2 (A and C) and 4 (B and D) hpf, showing centrosomal (γ-tubulin, green) and chromosomal (DAPI, red) distribution. E–H. anti-α-tubulin antibodies (green) and DAPI (red) double-stained embryos at 2 (E and G) and 4 (F and H) hpf. In the morphant embryos, γ-tubulin and microtubule organizations were severely affected and γ-TuSC formation impairment generated multinucleated blastomeres (arrowheads in D and H). I, J. Confocal projection images of α-tubulin-immunofluorescence staining of blastoderm in 8 hpf control and morphant embryos. Microtubule-stained spindle structures (arrowheads in I) and bundle organizations are shown. N, nucleus. K, L. DAPI-stained nuclei of control and tubg1-Mo embryos show a severe karyokinesis defect after γ-tubulin silencing. EXPRESSION / LABELING:
PHENOTYPE:
|
|
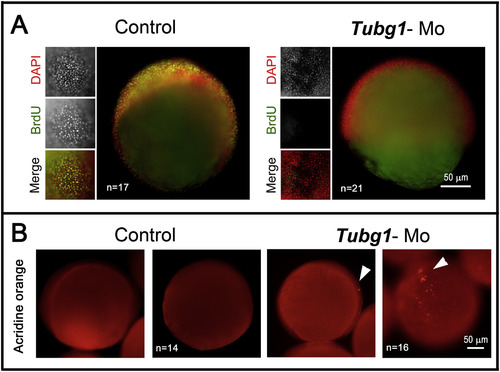
Cell proliferation arrest and apoptosis triggered in the absence of γ-TuSC formation. A. Zebrafish control and morphant embryos treated through the BrdU incorporation procedure. Embryos (8 hpf) were exposed to a BrdU pulse for 1 h, then fixed and treated for DNA labeling with DAPI (red) and immunolabeled for the incorporated BrdU (green). tubg1-Mo embryos did not incorporate BrdU in their DNA, indicating proliferation arrest. B. Apoptotic response revealed by TUNEL assay in embryos (8 hpf). The tubg1-morphant showed many clusters of cells positive for TUNEL stain (arrowheads) distributed throughout the blastoderm. PHENOTYPE:
|
Reprinted from Mechanisms of Development, 154, Pouchucq, L., Undurraga, C.A., Fuentes, R., Cornejo, M., Allende, M.L., Monasterio, O., γ-Tubulin small complex formation is essential for early zebrafish embryogenesis, 145-152, Copyright (2018) with permission from Elsevier. Full text @ Mech. Dev.