- Title
-
Pparα deficiency inhibits the proliferation of neuronal and glial precursors in the zebrafish central nervous system
- Authors
- Hsieh, Y.C., Chiang, M.C., Huang, Y.C., Yeh, T.H., Shih, H.Y., Liu, H.F., Chen, H.Y., Wang, C.P., Cheng, Y.C.
- Source
- Full text @ Dev. Dyn.
|
pparaa and pparab are expressed in the developing nervous system. A: real‐time polymerase chain reaction (RT‐PCR) analysis reveals that zebrafish pparaa and pparab transcripts are detected from 0.75 hpf to 10 hpf. pparaa displays relatively weak expression at 5.3, 8, and 10 hpf, and a high level of expression between 0.75 and 4.7 hpf, whereas pparab expression is detected at low levels at 8 and 10 hpf. Bottom bands show ef1α as a loading control. B: pparαa and pparαb expressions were analyzed using whole‐mount in situ hybridization. Abundant pparαa and pparαb expressions were observed in the embryo at 75% epiboly, bud, and 14‐somite stages. At 24 and 72 hpf, marked expressions were observed in the eyes (e), brain (b), spinal cord (sc), pharyngeal arches (pa), heart (h), and intestine (i). Staging of the embryos is shown in the bottom left corner of each panel. C: Embryos were sectioned sagittally through the neuroectoderm (i and iv) or transversely through the brain (ii, iii, v, and vi) after whole‐mount in situ hybridization. The regions of each section are indicated in (B). EXPRESSION / LABELING:
|
|
Pparα deficiency did not affect the formation of neural progenitors. A: GW6471 treatment or injection of pparaa ΔLBD or pparab ΔLBD reduced the expression of acox1, as examined by quantitative real‐time polymerase chain reaction (RT‐qPCR). B: Schematic of pparaa ΔLBD and pparab ΔLBD deletion constructs. DBD, DNA‐binding domain; LBD, ligand‐binding domain. C–J: GW6471 treatment (C,D), injection of pparaa ΔLBD or pparab ΔLBD, and coinjection of pparaa ΔLBD and pparab ΔLBD (E,F) did not alter the expression of neural progenitor marker sox2 and the anterior neural plate marker otx2b, as examined using whole‐mount in situ hybridization (C,E,G,I) and quantified through RT‐qPCR (D,F,H,J). Note that the expression of sox2 at 72 hpf was not affected by Pparα deficiency (G–J). The data represent three independent experiments. Values are expressed as the mean ± standard deviation. **P < 0.01; n.s. not significant. Staging of the embryos is shown in the bottom left corner of each panel in C and E. EXPRESSION / LABELING:
PHENOTYPE:
|
|
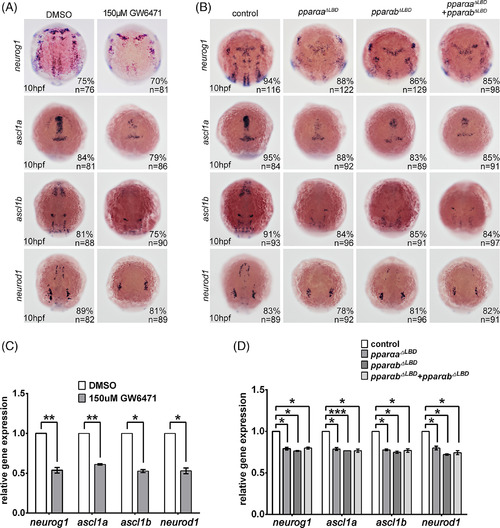
Pparα deficiency reduced neuronal precursors. A–D: In situ hybridization demonstrating the down‐regulation of neurog1, ascl1a, ascl1b, and neurod1 expression in embryos treated with GW6471, pparaa ΔLBD, pparab ΔLBD, or coinjection of pparaa ΔLBD and pparab ΔLBD (A,B), which was confirmed through quantitative real‐time polymerase chain reaction (C,D). * P < 0.05; **P < 0.01. Staging of the embryos is shown in the bottom left corner of each panel in A and B. |

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. |
|
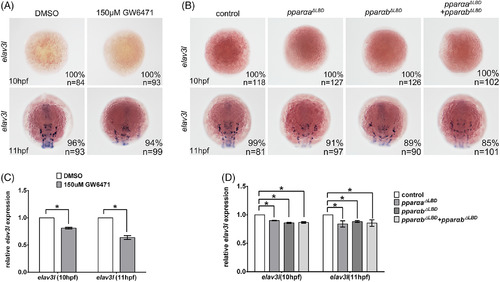
Pparα inactivation did not induce the premature differentiation of neuronal precursor cells. A–D: GW6471‐treatment, pparaa ΔLBD injection, pparab ΔLBD injection, or pparaa ΔLBD and pparab ΔLBD coinjection did not induce elval3 expression at 10 hpf or 11 hpf, as examined by in situ hybridization (A,B) and quantified by quantitative real‐time polymerase chain reaction (C,D). Note the decreased elval3 expression at 11 hpf in Pparα‐deficient embryos. Staging of the embryos is shown in the bottom left corner of each panel in A and B. EXPRESSION / LABELING:
PHENOTYPE:
|
|
PPARα deficiency inhibits the formation of glial precursors and radial glia. A–D: In situ hybridization (A,B) and quantitative real‐time polymerase chain reaction (C,D) demonstrating that the expression of slc1a3a and gfap was decreased in embryos treated with GW6471 or injected with pparaa ΔLBD or pparab ΔLBD. The embryo stages are displayed on the bottom left corner of each panel. *P < 0.05; **P < 0.01; n.s., not significant. EXPRESSION / LABELING:
PHENOTYPE:
|

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. EXPRESSION / LABELING:
PHENOTYPE:
|
|
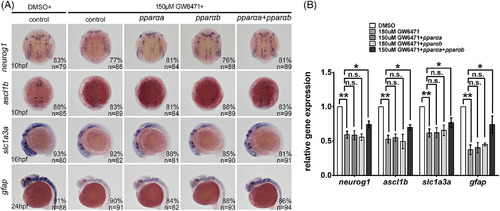
Pparαa or Pparαb alone is not sufficient to rescue the GW6471‐treatment phenotype. In situ hybridization and quantitative real‐time polymerase chain reaction demonstrating that injection of either zebrafish pparaa or pparab coding sequence could not rescue the downregulation of neuronal and glial markers caused by GW6471. In contrast, coinjection of pparaa and pparab rescued the neuronal and glial defects caused by GW6471. **P < 0.01; ***P < 0.001. Staging of the embryos is shown in the bottom left corner of each panel. |