- Title
-
EF-hand domain containing 2 (Efhc2) is crucial for distal segmentation of pronephros in zebrafish
- Authors
- Barrodia, P., Patra, C., Swain, R.K.
- Source
- Full text @ Cell Biosci.
|
Expression of efhc2 mRNA during zebrafish development. RT-PCR and whole embryo mRNA in situ hybridization (WISH) was carried out on different stages of zebrafish development. a RT-PCR analysis of efhc2 mRNA expression during zebrafish development. ef1α was used as loading control. b Ubiquitous expression of efhc2 at 6 hpf. c efhc2 expression in kupffer’s vesicle (KV) at 9 hpf. d Expression in the intermediate mesoderm (IM), notochord (NC) and otic vesicle (O) at 12 hpf. e Expression of efhc2 in olfactory placode (OP), pronephros (P), epiphysis (E), notochord (NC), otic vesicle (O) and in tailbud (TB) at 24 hpf. f Magnified image of zebrafish trunk show strong expression of efhc2 in PST and DE and low expression in PCT, DL and PD. g Dorsal view of 24 hpf embryos showing its expression in pronephros. h, i Two colour WISH of pdzk1 and efhc2. J Section through trunk showing efhc2 expression in pronephros (P). k, l Expression of efhc2 in epiphysis, pronephros, notochord, otic vesicle and in tail bud at 36 and 48 hpf. (M) Expression of efhc2 in neuromast cells and olfactory placode at 72 hpf EXPRESSION / LABELING:
|
|
Effect of Efhc2 knock-down on pronephros development and function. a Schematic representation of the binding of the splice-blocking efhc2 morpholino (efhc2-MO) targeting exon-3 and the forward and reverse primers used in RT-PCR to verify its effect. RT-PCR on RNA extracted from 24 hpf uninjected embryos or embryos injected with efhc2-MM control showed a 651 bp product as expected. The embryos injected with efhc2-MO showed a 311 bp fragment confirming the blocking of normal splicing of efhc2 by this morpholino. b efhc2-Mo morphants showed morpholino dose-dependent developmental defects as compared with wild-type and mismatch controls. Arrow indicates mild pericardial oedema and arrowhead indicates hydrocephalus. c Fluorescent 40 kDa dextran was injected into cardinal vain of control and efhc2-Mo morphants at 48 hpf. Accumulation of dextran in yolk and oedema shows defects in pronephros function in efhc2-Mo morphants at 72 and 96 hpf |
|
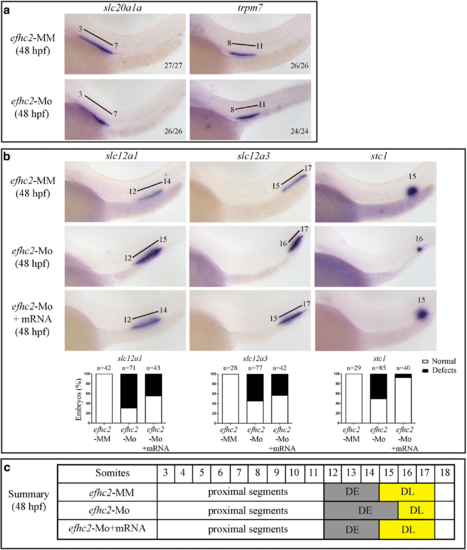
Characterizing the phenotype caused by Efhc2 knock-down. a and b WISH for pronephros segment specific genes on 48 hpf morpholino injected embryos. Expression of slc20a1a (PCT), trpm7 (PST), slc12a1 (DE), slc12a3 (DL) and stc1 (CS) in efhc2 mismatch control (efhc2-MM) and splice-blocking morpholino (efhc2-Mo) injected embryos. The phenotype caused by Efhc2 knock-down was rescued by co-injecting efhc2 mRNA along with efhc2 morpholino. c Schematic representation of defects caused by Efhc2 knock-down |
|
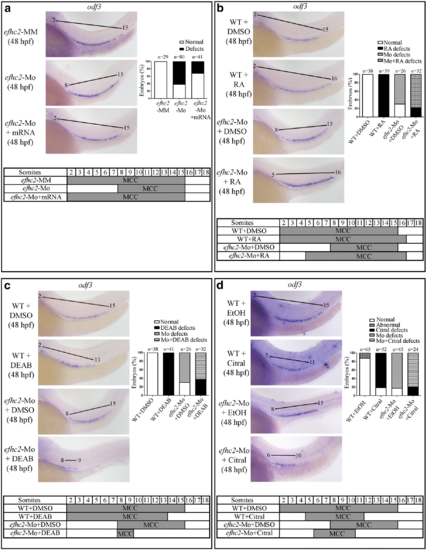
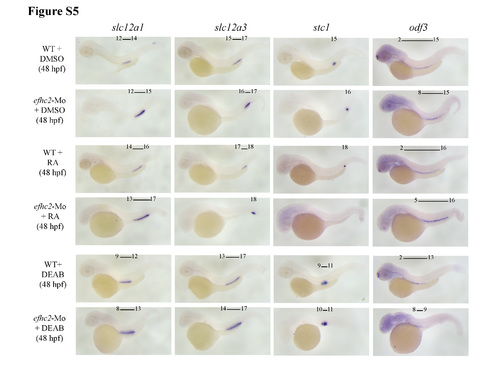
Role of Efhc2 in RA mediated segmentation of pronephros development. a Wild-type embryos or efhc2 morphants were treated with RA. Morphological changes caused by RA treatment in wild-type and efhc2 morphants. WISH showing expression of slc12a1 (DE), slc12a3 (DL) and stc1 (CS). Wild-type embryos or efhc2 morphants were treated with RA (1 × 10−7 M). efhc2 morphants treated with RA show expansion of expression domain of DE marker slc12a1 and almost or complete loss of DL and CS markers slc12a3 and stc1 as compared with wild-type embryos treated with RA. b Summary of the effects of RA on WT and efhc2 morphants at 48 hpf |
|
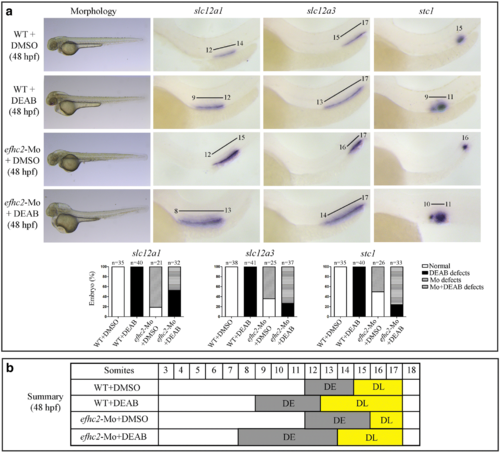
Effect of blocking RA synthesis by DEAB in efhc2-Mo morphants during pronephros segmentation. a Wild-type embryos or efhc2-Mo morphants were treated with DEAB (1 × 10−5 M). Morphological changes caused by DEAB treatment in wild-type and efhc2 morphants. efhc2-Mo morphants treated with DEAB show expansion of expression domain of DE marker slc12a1 and slight reduction of DL and CS markers slc12a3 and stc1 as compared with wild-type embryos treated with DEAB. b Summary of the effects of DEAB on WT and efhc2-Mo morphants at 48 hpf |
|
Effect of RA synthesis blocker citral on pronephros segmentation in efhc2-knocked-down embryos. a Wild-type embryos or efhc2-Mo morphants were treated with citral (2.5 × 10−5 M). Morphological defects caused by citral treatment in wild-type and efhc2 morphants. efhc2-Mo morphants treated with citral show expansion of expression domain of DE marker slc12a1 and slight reduction of DL and CS markers slc12a3 and stc1 as compared with wild-type embryos treated with citral. b Summary of the effects of citral on WT and efhc2-Mo morphants at 48 hpf |
|
Role of Efhc2 and RA in MCC development. a WISH for MCC specific marker odf3 in efhc2 mismatch control (efhc2-MM) and splice-blocking morpholino (efhc2-Mo) injected embryos. The phenotype caused by Efhc2 knock-down was rescued by efhc2 mRNA. b Wild-type embryos or efhc2-Mo morphants were treated with RA. WISH showing expression of odf3 gene. efhc2-Mo morphants treated with RA show reduction of expression domain of MCC as compared to wild-type embryos treated with RA. c Wild-type embryos or efhc2-Mo morphants were treated with DEAB. WISH showing expression of odf3 gene. efhc2-Mo morphants treated with DEAB show reduction of expression domain of MCC as compared to wild-type embryos treated with DEAB. d Wild-type embryos or efhc2-Mo morphants were treated with citral. WISH showing expression of odf3 gene. efhc2-Mo morphants treated with citral show reduction of expression domain of MCC as compared to wild-type embryos treated with citral |
|
Effect of Efhc2 knock-down on nephron segmentation. (A) Morphological defects seen by injection of efhc2-ATG-Mo. Arrow indicates mild pericardial oedema. (B) WISH showing expression of slc12a1 (DE), slc12a3 (DL), stc1 (CS) and odf3 (MCC) in efhc2-ATG-Mo injected and standard control injected embryos. (C) Summary of defects caused by Efhc2 translation blocking morpholino. |
|
Efhc2 knock-down results in nephron segmentation defects. (A) WISH for pronephros segment or MCC specific markers on 24 and 48 hpf morpholino injected embryos. Expression of slc20a1a (PCT), trpm7 (PST), slc12a1 (DE), slc12a3 (DL), stc1 (CS) and odf3 (MCC) in efhc2 mismatch control (efhc2-MM) and splice-blocking morpholino (efhc2-Mo) injected embryos. (B) Summary of defects caused by Efhc2 knock-down. |
|
Phenotype caused by over-expression of efhc2 mRNA. (A) efhc2 mRNA injected embryos showed dose-dependent pronephros defects. |
|
Role of RA and Efhc2 in pronephros segmentation. Wild-type embryos or efhc2-Mo morphants were treated with DMSO, RA and DEAB. WISH showing expression of slc12a1 (DE), slc12a3 (DL), stc1 (CS) and odf3 (MCC). efhc2-Mo morphants treated with RA show expansion of expression domain of DE marker slc12a1 and almost or complete loss of DL and CS markers slc12a3 and stc1 as compared with wild-type embryos treated with RA. The expression domain and the number of cells expressing MCC maker odf3 was partially rescued by RA treatment in the morphants. DEAB treated efhc2-Mo morphants show expansion of DE, DL, and CS as compared with wild-type embryos treated with DEAB. The expression domain of odf3 is reduced in DEAB treated WT embryos. |