- Title
-
γ-Aminobutyric acid receptor alpha 1 subunit loss of function causes genetic generalized epilepsy by impairing inhibitory network neurodevelopment
- Authors
- Samarut, É., Swaminathan, A., Riché, R., Liao, M., Hassan-Abdi, R., Renault, S., Allard, M., Dufour, L., Cossette, P., Soussi-Yanicostas, N., Drapeau, P.
- Source
- Full text @ Epilepsia
|
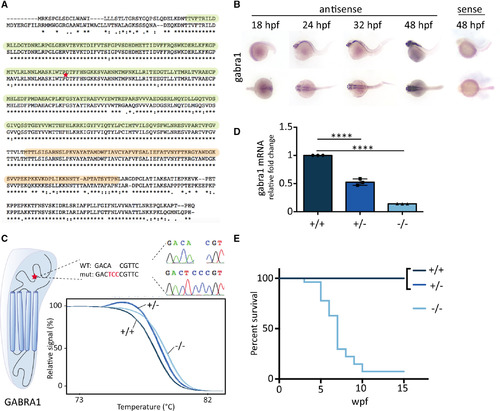
GABRA1 knockout leads to premature death in zebrafish. A, Human (top, ENST00000393943.9) and zebrafish (ENSDART00000100000.4) GABRA1 protein sequences were aligned using Clustal W algorithm, and amino acid identity is indicated by asterisks. Colons and dots indicate conservation between amino acid of strongly or weakly similar properties, respectively. The γ‐aminobutyric acid (GABA) binding and transmembrane domains are highlighted in green and orange, respectively. The CRISPR‐targeted site is indicated with a red star. B, Whole mount in situ hybridization against gabra1 mRNA showed a faint expression in the central nervous system at 18 hours postfertilization (hpf) that increased in the developing brain at 24 and 32 hpf. The expression was broad, strong, and restricted to the brain at 48 hpf. C, CRISPR mutagenesis of the GABRA1 GABA‐binding domain coding sequence led to the A>T+CC mutation (mut). The mutation was confirmed by Sanger sequencing. WT, wild‐type. D, Real‐time quantitative polymerase chain reaction analysis of RNAs from 5 days postfertilization larvae (in triplicate with n = 7) shows a significant decrease of gabra1 mRNA expression in gabra1+/− (52.52% of WT ± 0.0327) and gabra1−/− (14.04% of WT ± 0.00058) larvae when compared to their WT siblings (one‐way analysis of variance and Tukey multiple comparison test; ****P < 0.0001). E, The progeny of gabra1+/− individuals was genotyped by tail‐clipping at 1 week postfertilization (wpf), raised separately according to their genotypes, and the survival of each class (+/+, +/−, −/−; n = 8) was monitored every week until 15 weeks of age. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Gabra1−/− fish undergo evident generalized seizures upon light. A, Distance swam over 8 hours in the dark (left) or light (right) by 5 weeks postfertilization (wpf) gabra1+/− or −/− juveniles revealed significant hypoactivity of gabra1−/− compared to their heterozygous siblings (sib) in both light (t test, P < 0.03) and dark cycles (t test, P < 0.003). B, Measurements of the distance swam per 30‐second period after light was turned on revealed a strong increase of 5.5 wpf gabra1−/− motility just after light was turned on and followed by a period of immobility. C, Video frames of juvenile (5‐6 wpf) gabra1+/+ (top), +/− (middle), and −/− (bottom) siblings upon light exposure. Immediately after light, gabra1−/− fish underwent a first tonic‐like phase characterized by arching of the body (arrows), uncontrolled twitching (as seen with the red tracks), and loss of posture (arrowheads). After this first phase, which lasted a few seconds, gabra1−/− fish underwent a second, clonic‐like phase, during which they underwent rapid and uncontrolled movements, fast muscle contractions, and whole‐body convulsions leading to a whirlpool swimming pattern (tracks in red). Lastly, after about 1 minute of seizure, they entered a third, postictal phase, during which they stayed immobile and breathed heavily for 3‐10 minutes. D, E, Maximum acceleration upon light of 4 days postfertilization (dpf) gabra1 embryos (n = 96) showing an increased startle response to light of gabra1−/− specifically. Tracks of 3 seconds following light exposure show a stereotyped pattern of +/+ (left), +/− (middle), and −/− (right) light response PHENOTYPE:
|
|
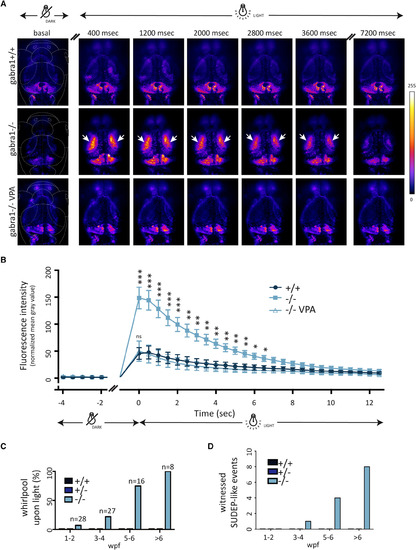
Generalized neuronal activity in gabra1−/− following light exposure and sudden death following generalized seizures. A, Gabra1+/− and NeuroD:GCaMP6f +/− were intercrossed, and neuronal activity was monitored under a confocal microscope at 8 days postfertilization. After a period of 30 minutes in complete darkness, switching on the laser induced a broad neuronal activity in both optic tecta in gabra1−/− (n = 12) compared to gabra1+/+ siblings (n = 10) that lasted for few seconds (arrows). The intense neuronal activity of −/− larvae was rescued to wild‐type (WT) level after exposure to valproic acid (VPA; 50 μmol/L, n = 7). B, Fluorescence quantification in the optic tecta before and after laser exposure shows a significant increase of fluorescence in −/− larvae compared to +/+ siblings. Each point corresponds to the relative quantification of fluorescence from a frame with 400‐millisecond exposure. VPA treatment fully rescues the fluorescence back to the WT level (Student t test: *P < 0.05, ** P < 0.01, ***P < 0.005). C, The whirlpool onset (ie, clonic‐like phase) increased with age, with a full penetrance at 6 weeks postfertilization (wpf) onward. D, Sudden unexpected death in epilepsy (SUDEP) events were quantified and occurred from 4 wpf onward only in gabra1−/− mutants. Of note is that data shown here correspond to the actual SUDEP events witnessed, but it is very likely that it occurs in many more fish PHENOTYPE:
|
|
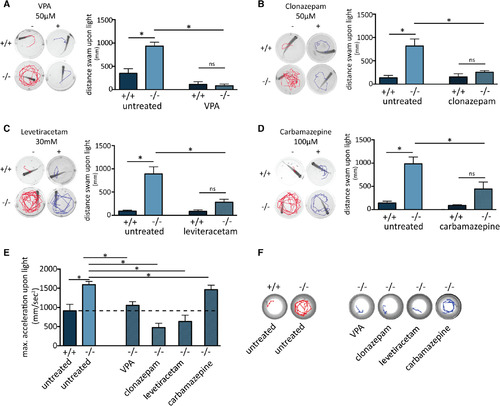
Differential attenuation of seizures by known antiepileptic drugs (AEDs). Five to 6 weeks postfertilization wild‐type (+/+, n = 5) or homozygous (−/−, n = 5) fish were incubated overnight with valproic acid (VPA; A), clonazepam (B), levetiracetam (C), or carbamazepine (D). Both steps of the light‐triggered seizure were analyzed before and after the treatment using the track of single fish for 3 seconds following light being switched on (left panels), as well as by quantifying the distance swam during the minute following light being turned on (right panels). E, The increased acceleration of 4 days postfertilization (dpf) gabra1−/− embryos was differentially alleviated by the four tested AEDs (n = 22 per assay). F, Stereotyped tracks of 4 dpf untreated gabra1−/− embryos and mutant embryos treated with the four AEDs. All drug treatments were performed overnight with a final concentration of either 50 μmol/L VPA, 50 μmol/L clonazepam, 30 mmol/L levetiracetam, or 100 μmol/L carbamazepine. ns, not significant. *P value <0.05 ... hide PHENOTYPE:
|
|
Whole transcriptome deep sequencing revealed 460 genes differentially expressed in gabra1−/− brains, although brain structure is not altered. A, Immunolabeling of 4 days postfertilization (dpf) whole gabra1 |
|
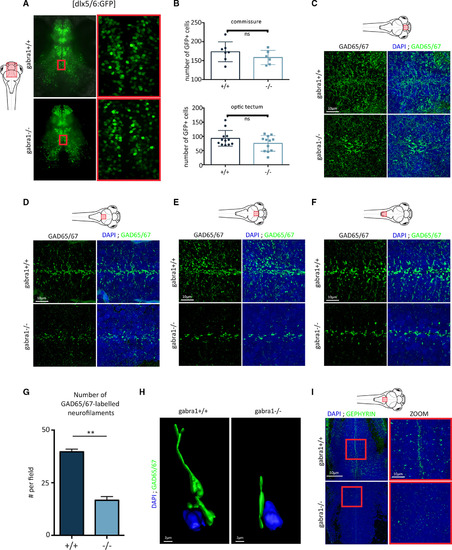
Reduction of inhibitory synaptic connectivity in gabra1−/− mutants. A, Confocal imaging of 8 days postfertilization (dpf) larval brains from gabra1 × dlx5/6:GFP transgenic lines. B, Quantification of the number of green fluorescent protein (GFP)+ cells at the commissure (top) or projecting to the optic tectum (bottom). ns, not significant. C‐F, Fluorescent immunodetection of GAD65/67 (green) combined with 4,6‐diamidino‐2‐phenylindole (DAPI) labeling on cryostat sections of different brain regions from 6 dpf gabra1+/+ and gabra1−/− zebrafish embryos (scale bars = 10 μm): telencephalon (C), anterior part of optic tectum (D), posterior part of optic tectum (E), spinal cord (F). G, Quantification of GAD65/67‐labeled neurofilaments (n = 3, Student t test: **P = 0.0019). H, Imaris‐reconstructed three‐dimensional image with Gad65/67 shown in green and the nucleus (DAPI) shown in blue. Scale bar = 2 μm. I, Sagittal sections of 6 dpf gabra1+/+ and gabra1−/− embryos hybridized with an antibody directed against postsynaptic density gephyrin (green) and nuclei stained with DAPI (blue; scale bar = 50 μm). Magnified views of the regions boxed in white and red rectangles are shown (scale bar = 10 μm). A decreased accumulation of gephyrin expression was observed in gabra1−/− mutants. For each imaging, the same field of observation is shown for +/+ and −/− larvae, thus avoiding regional bias ... hide PHENOTYPE:
|