- Title
-
Cre/lox-controlled spatio-temporal perturbation of FGF signaling in zebrafish
- Authors
- Kirchgeorg, L., Felker, A., van Oostrom, M., Chiavacci, E., Mosimann, C.
- Source
- Full text @ Dev. Dyn.
|
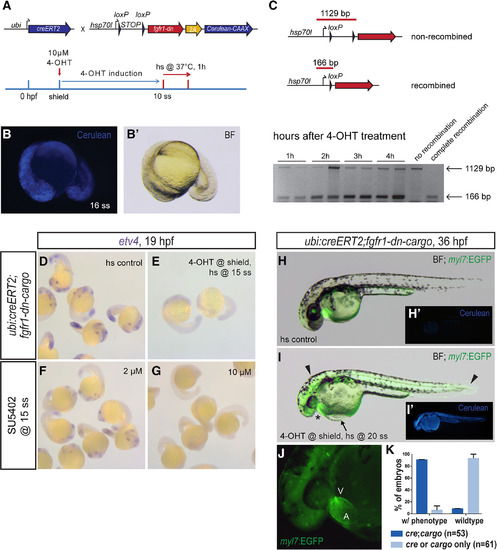
Global FGF signaling perturbation using the fgfr1‐dn‐cargo transgenic line. A: Schematic showing the crosses and a representative treatment scheme for ubiquitous genetic FGF signaling perturbation with the fgfr1‐dn‐cargo transgenic line. Note that fgfr1‐dn‐cargo contains a 2A‐linked Cerulean‐CAAX ORF. B,B’: Ubiquitous fgfr1‐dn‐cargo activation in 4‐OHT– and heatshock‐treated ubi:creERT2;fgfr1‐dn‐cargo transgenic embryos as shown in schematic (A) leads to ubiquitous but mosaic Cerulean‐CAAX (Cerulean) expression. C: CreERT2‐mediated ubiquitous excision of the loxP‐flanked STOP cassette in ubi:creERT2;fgfr1‐dn‐cargo transgenics 4‐OHT induced during early somitogenesis as detected by PCR (166 bp after recombination, 1129 bp when unrecombined). Excision of the STOP cassette occurs within 1 hr and gradually increases up to 4 hr after 4‐OHT treatment. Shown are PCR on recombined and unrecombined transgene insertions. D–G: Heatshock (hs) controls, ubi:creERT2;fgfr1‐dn‐cargo embryos induced with 4‐OHT at shield stage and heatshocked at 15 ss, and wild‐type embryos treated with 2 or 10 µM SU5402 at 15 ss were fixed at 19 hpf and stained for etv4 expression via mRNA in situ hybridization. Ubiquitous fgfr1‐dn‐cargo activation and concentration‐dependent SU5402 treatment abolish etv4 expression as a readout for FGF signaling activity. H,I: Overlay of EGFP expression on a brightfield (BF) image of a 36‐hpf heatshock control and ubi:creERT2;fgfr1‐dn‐cargo embryo induced with 4‐OHT at shield stage and heatshock‐treated at 20 ss. Embryos with ubiquitous expression of Fgfr1‐dn during late somitogenesis have a mis‐looped heart (asterisk) as well as head defects and malformations in posterior tail structures (arrowheads). The heart malformations also manifest in blood pooling in front of the inflow tract of the heart, leading to a visible edema on top of the yolk (arrow). H’,I’: Cerulean‐CAAX expression in control and signaling‐perturbed embryos. J: 7x magnification of the heart of a 4‐OHT–induced and heatshock‐treated ubi:creERT2;fgfr1‐dn‐cargo double transgenic (I) showing heart defects with a large atrium (A) and diminished ventricle (V); green background fluorescence is caused by bleedthrough of Cerulean fluorescence (see also I’). K: Quantification of phenotypes resulting from global FGF signaling perturbation in genetically perturbed double‐transgenic ubi:creERT;fgfr‐dn‐cargo embryos and single‐transgenic heatshock controls treated as indicated (I). |
|
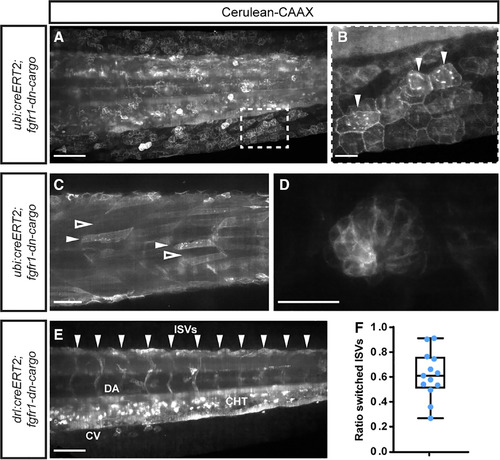
Recombination efficiency of fgfr1‐dn‐cargo. A–F: SPIM imaging of 3‐dpf ubi:creERT2;fgfr‐dn‐cargo embryos, 4‐OHT induced at shield stage and heatshocked at 3 dpf shortly before imaging; signal shows Cerulean‐CAAX fluorescence of the fgfr1‐dn‐cargo transgene upon successful loxP recombination. A,B: Lateral view of tail, showing the spectrum of Cerulean‐CAAX–expressing clones, with hatched outline magnified (B) to document the Cerulean‐CAAX signaling confines to membranes and perinuclear ER (arrowheads) of successfully recombined cells. C,D: Examples for recombination efficiency in ubi:creERT2;fgfr‐dn‐cargo embryos with Cerulean‐CAAX–positive and –negative clones, including somitic muscle fibers (C, white arrowhead for positive fibers, black arrowhead for negative fibers) and a lateral‐line neuromast (D). E,F: Quantification of recombination efficiency using Cerulean‐CAAX lineage labeling in intersomitic vessels (ISVs) in drl:creERT2;fgfr‐dn‐cargo embryos following 4‐OHT treatment at shield stage and heatshock at 3 dpf. E: Cerulean‐CAAX fluorescence was counted in ISVs (arrowheads indicating positions), unilateral or bilateral signal counted as positive labeling. F: Ratio of labeled vs. total ISV spaces as proxy for recombination efficiency (n = 13); box depicts standard deviation, bars the maximum spread. Scale bars A,C,E = 100 µm. Scale bars B,D = 20 µm. |
|
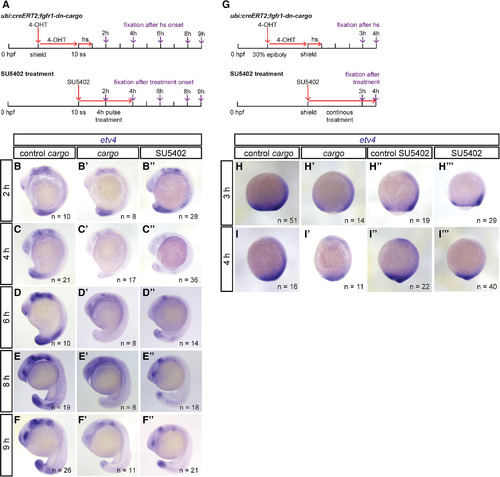
Temporal dynamics of genetic FGF signaling perturbation using fgfr1‐dn‐cargo. A: Schematic showing the time line of treatments for genetic and chemical FGF signaling perturbations and time points of embryo fixation for signaling activity readouts. Red arrows indicate time points and duration of drug (10 µM 4‐OHT and 5 µM SU5402) and heatshock treatments; violet arrows mark time points of embryo fixation after treatment onset (heatshock or SU5402 application). B–F: Representative embryos stained for etv4 mRNA expression via in situ hybridization in Cerulean‐negative single‐transgenic controls (control cargo), ubi:creERT2;fgfr1‐dn‐cargo (cargo), and SU5402‐treated embryos; lateral views, anterior to the top. Number “n” indicates individual embryos stained and analyzed for each condition. B,C: After FGF inhibition was initiated at mid‐somitogenesis (10 ss), etv4 expression in ubi:creERT2;fgfr1‐dn‐cargo and SU5402‐treated embryos was decreased 2 hr and absent 4 hr after treatment onset. D‐F: etv4 expression started recovering 2 hr after SU5402‐treatment was stopped (6 hr after initiation of a 4‐hr pulse) and 8 hr after heatshock in ubi:creERT2;fgfr1‐dn‐cargo double transgenics; 9 hr after treatment onset, etv4 expression recovered to a large extent in genetically and pulse‐treated chemically perturbed embryos (F). G–I: FGF signaling perturbation during gastrulation stages. G: Schematic showing the time line of treatments (as in A). H,I: Representative embryos stained for etv4 mRNA expression with in situ hybridization in Cerulean‐negative single‐transgenic controls (control cargo), Cerulean‐positive ubi:creERT2;fgfr1‐dn‐cargo double transgenics (cargo), untreated wild‐type controls (control SU5402), and SU5402‐treated embryos; lateral views, anterior to the top. Number “n” indicates individual embryos stained and analyzed for each condition. etv4 expression in ubi:creERT2;fgfr1‐dn‐cargo and SU5402‐treated embryos was decreased 3 hr and 4 hr after treatment at shield stage, but never completely lost as at later stages (see also C,D). Note that Cerulean‐CAAX expression could not be observed 1–2 hr post‐heatshock, thus etv4 expression analysis is only shown after 3 hr. |
|
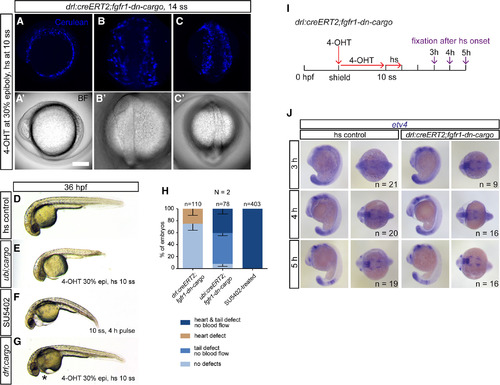
Tissue‐specific FGF signaling perturbation in the developing LPM. A–C: Maximum‐intensity projection and brightfield images of an in toto SPIM‐imaged drl:creERT2;fgfr1‐dn‐cargo double‐transgenic embryo. The cell membrane of all Fgfr1a‐dn–expressing cells is fluorescently labeled with Cerulean‐CAAX after heatshock treatment, revealing mosaic transgene expression throughout the developing LPM; (A) lateral view, anterior to the top; (B) dorsal view of the anterior embryo, anterior to the top; (C) dorsal view of the posterior embryo, anterior to the top. D–G: Brightfield images of global or tissue‐specific FGF signaling–perturbed embryos, lateral views, anterior to the left. E,F: Ubiquitously Fgfr1‐dn–expressing or SU5402‐treated embryos show severe cardiovascular defects accompanied by body axis shortening and defects in posterior tail formation. G: Selective cardiovascular phenotypes (asterisk) with no apparent body axis deformations are apparent after tissue‐specific FGF signaling perturbation in drl–expressing descendants. H: Quantifications of phenotypes observed after drl:creERT2;fgfr1‐dn‐cargo‐, ubi:creERT2;fgfr1‐dn‐cargo‐, or SU5402‐mediated FGF signaling inhibition. Number “n” indicates the number of individual embryos analyzed per condition; “N” indicates the number of individual experiments performed. I–J: Embryo‐wide FGF target gene expression remains grossly intact upon LPM‐specific FGF signaling perturbation. I: Schematic showing the time line of treatments for genetic FGF signaling perturbations in drl:creERT2;fgfr1‐dn‐cargo and time points of embryo fixation for signaling activity readouts. Red arrows indicate time points and duration of 4‐OHT and heatshock treatments; violet arrows mark time points of embryo fixation after initiation of transgene activation via heatshock. J: Embryos selectively perturbed for FGF signaling in the drl descendants at somitogenesis displayed no detectable differences in etv4 expression when compared to unperturbed siblings 3–5 hr after heatshock treatment (lateral and dorsal views, anterior to the left). Number “n” indicates number of individual embryos analyzed for each condition. |
|
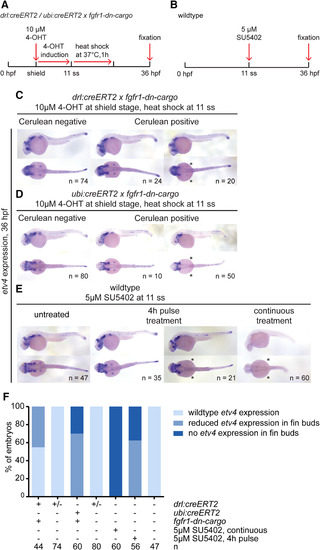
etv4 expression in tissue‐specific and global FGF signaling–perturbed embryos at 36 hpf. A,B: Transgenic embryos were treated with 4‐OHT at shield stage and heatshock‐treated at 11 ss (A), while wild‐type embryos were treated with SU5402 either continuously or for a 4‐hr pulse for comparison. B: Expression of the FGF target gene etv4 assayed at 36 hpf. C–E: etv4 expression in the different conditions. etv4 expression was grossly unaffected by LPM‐specific perturbation using drl:creERT2 priming fgfr1‐dn‐cargo (C); compare Cerulean‐negative to Cerulean‐positive, fgfr1‐dn–expressing embryos, with notable exception of pectoral fin expression that was absent in a cohort of fgfr1‐dn–expressing embryos (asterisks). etv4 was also grossly unaffected after ubiquitous FGF perturbation in cohorts of ubi:creERT2;fgfr1‐dn‐cargo embryos (D) and SU5402 pulse‐treated embryos (E), while pectoral fin expression was again affected (asterisks in D,E). Continuous FGF signaling inhibition with SU5402 per indicated conditions (B) broadly inhibited etv4 expression (asterisks in E). F: Quantification of etv4 expression in embryos subjected to embryo‐wide or tissue‐specific signaling perturbations. Number “n” indicates number of individual embryos analyzed for each condition. |
|
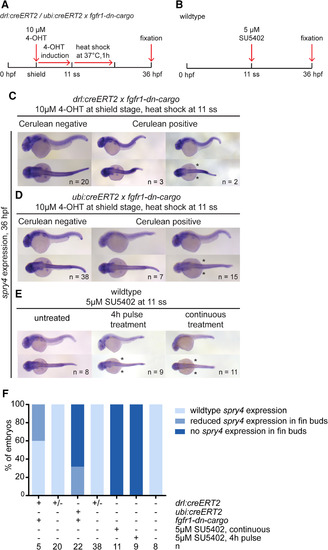
spry4 expression in tissue‐specific and global FGF signaling–perturbed embryos at 36 hpf. A,B: Transgenic embryos were treated with 4‐OHT at shield stage and heatshock‐treated at 11 ss. Wild‐type embryos were treated with SU5402 either continuously or for a 4‐hr pulse. C–E: spry4 expression was completely absent in embryos continuously treated with SU5402, and reduced or absent in fin buds of transgenic (drl‐based LPM priming or ubi‐based ubiquitous priming) or pulse‐treated embryos; lateral and dorsal views, anterior to the left; asterisks indicate loss of pectoral fin expression. F: Quantification of spry4 expression in embryos subjected to embryo‐wide or tissue‐specific signaling perturbations. Number “n” indicates number of individual embryos analyzed for each condition. |
|
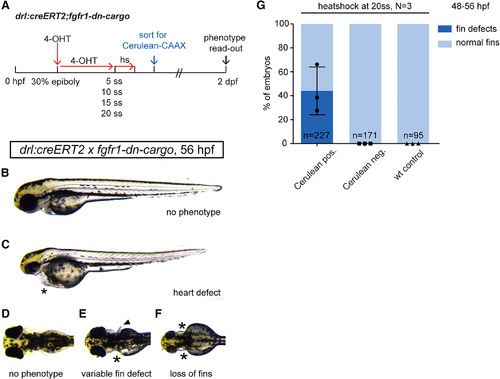
Temporal dissection of tissue‐specific FGF signaling control of cardiac and fin development. A: Schematic showing experimental time line of tissue‐specific FGF signaling perturbations at discrete developmental stages. 4‐OHT induction at 30% epiboly triggers Cre/lox recombination in earliest drl‐expressing progenitors. Heatshock treatments at distinct time points throughout somitogenesis target different phases of cardiac and fin development. B–D: Phenotypes scored in 2‐dpf drl:creERT2;fgfr1‐dn‐cargo embryos treated as indicated in the schematic above. B,C: Heart defects as scored through blood pooling and cardiac edema on top of the yolk (asterisks); lateral views, anterior to the top. D–F: Fin defects were counted upon unilateral; or bilateral fin deformations (arrow head in E, compare to normal pectoral fins in D) and/or loss (asterisks in E,F). G: Quantifications of phenotypes in drl:creERT2;fgfr1‐dn‐cargo double transgenics heatshock‐treated at different time points during somitogenesis. LPM‐specific FGF perturbation triggered by heatshock at 20 ss results in pectoral fin defects in on average 44.09% (s.d. 20.01, p = 005, total n = 227, three independent experiments) of double‐transgenic embryos with prior Cerulean expression; earlier heatshock timings caused no discernible fin defects. Number “n” indicates the number of individual embryos analyzed per condition; N indicates the number of individual experiments performed. Statistics based on one‐way ANOVA, multiple‐comparison Tukey's post‐test. |