- Title
-
Sensing of cytosolic LPS through caspy2 pyrin domain mediates noncanonical inflammasome activation in zebrafish
- Authors
- Yang, D., Zheng, X., Chen, S., Wang, Z., Xu, W., Tan, J., Hu, T., Hou, M., Wang, W., Gu, Z., Wang, Q., Zhang, R., Zhang, Y., Liu, Q.
- Source
- Full text @ Nat. Commun.
|
Caspase-5-like activity is essential for pyroptosis in zebrafish fibroblasts. a ZF4 zebrafish fibroblasts were infected with wild-type (EIB202) or 0909I E. piscicida for 2 h at a multiplicity of infection (MOI) of 50, or left uninfected. Supernatants from the indicated ZF4 cells were analyzed for cell death, as measured by lactate dehydrogenase (LDH) release. b ZF4 cells were infected with 0909I E. piscicida for 2 h at an MOI of 50. Relative caspase activity was then measured by incubating cell lysates with fluorogenic and chromogenic substrates of caspase-1 (YVAD), caspase-2 (VDQQD), caspase-3/7 (DEVD), caspase-4 (LEVD), caspase-5 (WEHD), caspase-8 (IETD), and caspase-9 (LEHD). c, d ZF4 cells were treated with caspase-3/7, pan-caspase, caspase-1, caspase-4, and caspase-5 inhibitors (Ac-DEVD-CHO, Z-VAD-FMK, Z-YVAD-FMK, Ac-LEVD-CHO, and Z-WEHD-FMK, respectively). c LDH release for cell death was measured 2 h after 0909I E. piscicida infection. d Images were taken after 0909I E. piscicida infection for 2 h. Propidium iodide (PI) was added to detect the loss of plasma membrane integrity. Arrows indicate cells exhibiting pyroptotic-like features. Scale bar, 50 µm. e–g ZF4 cells were primed with Pam3CSK4 for 4 h, before being stimulated with cholera toxin B subunit (CTB) plus LPS, LPS, or CTB alone for 12 h. e Supernatants from the indicated ZF4 cells were analyzed for cell death, as measured by lactate dehydrogenase (LDH) release. f Cytosolic LPS-delivered ZF4 cells were treated with indicated caspase inhibitors as in Fig. 1c, Supernatants from the indicated ZF4 cells were analyzed for cell death, as measured by lactate dehydrogenase (LDH) release. g Relative caspases activity was measured by incubating cell lysates with indicated fluorogenic and chromogenic substrates as in Fig. 1b. a–g Results are representative of at least three independent experiments, and error bars denote the SD of triplicate wells. *p < 0.05 (t test) |
|
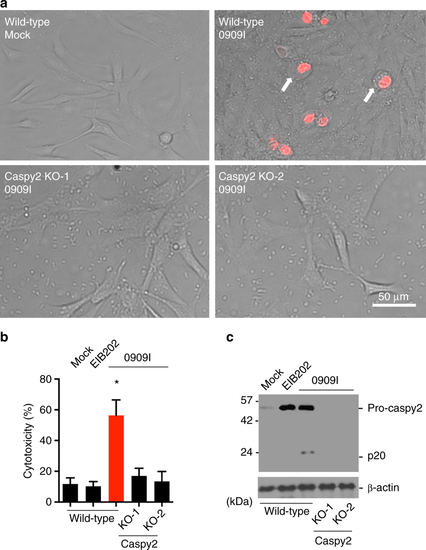
Caspy2 plays a critical role in pyroptosis in zebrafish fibroblasts. a–c Wild-type and caspy2-knockout (KO) ZF4 cells were infected with EIB202 or 0909I E. piscicida for 2 h at an multiplicity of infection (MOI) of 50, or left uninfected. a Images were taken as in Fig. 1d. Propidium iodide (PI) was added to detect the loss of plasma membrane integrity. Arrows signify cells with pyroptotic-like features. Scale bar, 50 µm. b Supernatants from the indicated ZF4 cells were analyzed for cell death measured by lactate dehydrogenase (LDH) release. c Mixtures of cell lysates and supernatants were subjected to immunoblotting. (a–c) Results are representative of at least three independent experiments, and error bars denote the SD of triplicate wells. *p < 0.05 (t test) |
|
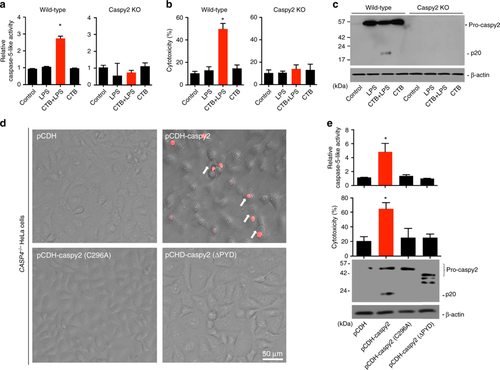
Caspy2 directly binds to LPS to induce its oligomerization. a Streptavidin pulldown assays of the binding of biotin-conjugated LPS and Pam3CSK4 to purified HA-tagged caspy2, caspy2 (C296A), caspy2 (ΔPYD), caspy2 PYD, caspy and caspy PYD in transfected HEK293T cells. Shown are anti-HA immunoblots of pulled down proteins and total lysates (input). b Purified HA-tagged caspy2 and caspy2 PYD in transfected HEK293T cells were incubated with LPS or Pam3CSK4. The samples were analyzed by the pore-limited native gel electrophoresis. c Wild-type and caspy2-KO ZF4 cells were infected with wild-type (EIB202) or 0909I E. piscicida (with/without 10 mM glycine) for 2 h at a multiplicity of infection (MOI) of 50. Confocal laser scanning microscopic analysis of nuclei (2-(4-amidinophenyl)-1H-indole-6-carboxamidine (DAPI), blue) and caspy2 foci (green, white arrowheads). Direct interference contrast (DIC)/phase images are shown. Scale bar, 10 µm. Statistics of the percentages of cells showing signals for caspy2 foci are listed below. (Approximately 200 cells were counted in each sample. Mean ± SD of triplicate samples.) d Wild-type and caspy2-KO ZF4 cells were infected with wild-type (EIB202) or 0909I E. piscicida (with/without 10 mM glycine) for 2 h at an MOI of 50, or left untreated (Mock). The samples were analyzed by the pore-limited native gel electrophoresis and immunoblotting (upper panel). Cell lysates and DSS cross-linked pellets from wild-type and caspy2-KO ZF4 cells treated as indicated were analyzed by immunoblotting for caspy2 oligomerization (lower panel). WB western blot. Results are representative of at least three independent experiments |
|
Intracellular LPS-triggered-caspy2 noncanonical inflammasome activation. a-c ZF4 cells were primed with Pam3CSK4 for 4 h, before being stimulated with cholera toxin B subunit (CTB) plus LPS, LPS, or CTB alone for 12 h. Relative caspase-5-like activity was measured by incubating cell lysates with fluorogenic and chromogenic caspase-5 substrates (WEHD) (a). Supernatants from the indicated HeLa cells were analyzed for cell death, as measured by lactate dehydrogenase (LDH) release (b). Mixtures of cell lysates and supernatants were subjected to immunoblotting (c). d, e CASP4−/− HeLa cells were transduced with a vector expressing wild-type caspy2, caspy2 (C296A), caspy2 (ΔPYD), or the empty vector. Cells were primed with LPS for 4 h, before being stimulated with CTB plus LPS for 12 h. Images were taken as in Fig. 1d (d). Propidium iodide (PI) was added to detect the loss of plasma membrane integrity. Arrows denote cells with pyroptotic-like features. Scale bar, 50 µm. Relative caspase-5-like activity and LDH release assays were conducted as in a and b (e). Immunoblotting for the caspy2 forms indicated is shown. a–e Results are representative of at least three independent experiments, and error bars denote the SD of triplicate wells. *p < 0.05 (t test) |
|
Pattern of caspy2 expression in zebrafish. a Reverse transcription polymerase chain reaction (RT-PCR) analysis of relative caspy2 expression at various stages of development. b Immunoblotting analysis of caspy2 levels at various stages of whole larvae development. c Whole-mount in situ hybridization targeting caspy2 mRNA in zebrafish at the developmental stages indicated. Black arrowheads, mouth; white arrowheads, pharyngeal arches; red arrowheads, gut. d RT-PCR analysis of relative caspy2 expression in the indicated adult zebrafish organs. a–d Results are representative of at least three independent experiments EXPRESSION / LABELING:
|
|
Caspy2 restricts bacterial colonization of the zebrafish gut in vivo. a–e Control-morpholino oligonucleotide (MO) or caspy2-MO zebrafish larvae at 5 days postfertilization (dpf) were infected by immersion with 105 colony forming units (CFU)/ml of 0909I E. piscicida. a Zebrafish larvae (5 dpf) were infected by immersion with wild-type (EIB202) or 0909I E. piscicida. Immunoblotting analysis of caspy2 levels at the indicated post-infection time points. b Survival of zebrafish larvae was monitored for 4 days. (n = 60 for control-MO, n = 60 for caspy2-MO). Results are representative of at least three independent experiments, **p < 0.01 (log-rank). c Zebrafish larvae were collected at the indicated postinfection time points, and homogenates were made for CFU counts. Each symbol represents the average counts of five larvae (n = 100 for control-MO, n = 100 for caspy2-MO). Results are representative of at least three independent experiments. *p < 0.05 (ANOVA). d Images of infected zebrafish larvae. Green fluorescent protein (GFP)-0909I E. piscicida. e Representative images of hematoxylin and eosin (H&E) staining of gut sections from 0909I-infected control-MO or caspy2-MO zebrafish larvae. Square frame, intestinal wall morphology; Scale bar, 10 µm |
|
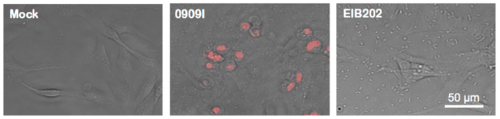
0909I E. piscicida infection induces pyroptosis in ZF4 cells. Images of uninfected ZF4 zebrafish fibroblasts and those infected with wild-type (EIB202) or 0909I E. piscicida for 2 h at a multiplicity of infection (MOI) of 50. Propodium iodide (PI) was added to detect loss of plasma membrane integrity. Results are representative of at least three independent experiments. Scale bar, 50 μm. |
|
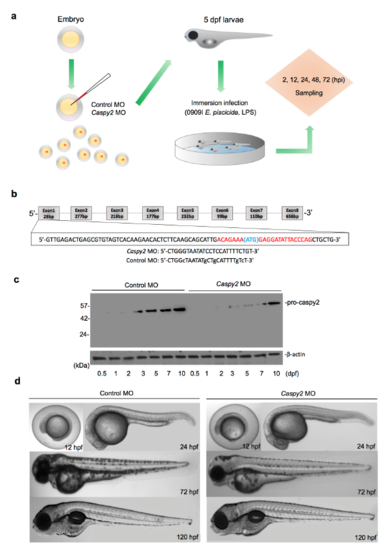
Representation of the zebrafish larvae immersion Infection Model. (a) The zebrafish microinjection and immersion infection procedures. (b) Generation of caspy2 morphant zebrafish by morpholino oligonucleotide (MO)-mediated knockdown. The upper diagram shows the region of caspy2 targeted to block translation. (c and d) Analysis of the phenotypes of caspy2-MO- and control-MO-injected larvae. (c) Immunoblotting of caspy2 expression in morphants and controls up to 5 days post fertilization (dpf). (d) Representative images of the phenotypes of caspy2-MO- and control-MO-injected zebrafish up to 120 h post fertilization (hpf). (c and d) Results are representative of at least three independent experiments. |
|
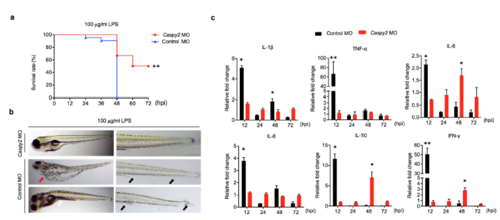
Caspy2 is critical for lethal sepsis in zebrafish larvae. (a-c) Controlmorpholino oligonucleotide (MO) or caspy2-MO zebrafish larvae at 5 dpf were immersed in 100 μg/ml of LPS. (a) Survival was then monitored for 72 hpi. Results are representative of at least three independent experiments. ** p < 0.01 (log-rank). (b) Distinct pathologies associated with lethal dose of LPS. Pericardial edema (red arrow); Fin erosion and ulceration (black arrow); Protrusions on the trunk or tail regions (white arrow). Results are representative of at least three independent experiments. (c) RT-PCR analysis of the specified cytokine transcripts at the indicated time points in control- and caspy2-MO larvae. Each bar represents mean results SEM from 3-4 pools of 15 larvae. RT-PCR for each pool was carried out using technical duplicates. *p < 0.05, **p < 0.01 (t-test). |