- Title
-
Zebrafish mutants and TEAD reporters reveal essential functions for Yap and Taz in posterior cardinal vein development
- Authors
- Astone, M., Lai, J.K.H., Dupont, S., Stainier, D.Y.R., Argenton, F., Vettori, A.
- Source
- Full text @ Sci. Rep.
|
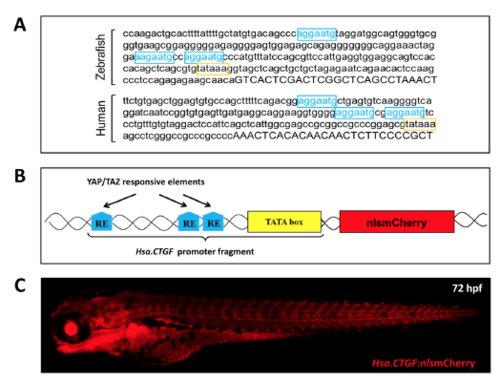
Hsa.CTGF-based Yap1/Taz zebrafish reporter line. (A) ctgf promoter contains three Yap1/Taz responsive elements (RE) and is remarkably conserved between zebrafish and humans. The promoter region upstream of the 5′UTR (uppercase) of the zebrafish ctgfa and human CTGF genes contain each three TEAD DNA-binding sites (YAP1/TAZ REs; in light blue) just before the TATA box (in yellow). Their sequences, orientation and distances are remarkably conserved between zebrafish and humans. (B) Schematic representation of the Hsa.CTGF-based Yap1/Taz reporter construct. The Yap1/Taz-responsive fragment of the construct is derived from the human CTGF promoter. It contains the pathway-specific REs MCAT and the TATA box, and regulates the expression of the downstream reporter gene. (C) Overview of a Tg(Hsa.CTGF:nlsmCherry) larva. |
|
Hsa.CTGF-based zebrafish transgenic lines are bona fide Yap1/Taz reporter. (A) Morpholino-mediated Yap1/Taz knockdown reduces Hsa.CTGF reporter signal (in red). Two splice morpholinos, targeting respectively Yap1 and Taz pre-mRNAs, were co-injected in one-cell stage Tg(Hsa.CTGF:nlsmCherry)ia49 embryos. (B–D) Constitutive activation of Yap1/Taz-mediated transcription increases Hsa.CTGF reporter signal (in red). The mRNA coding for constitutively active versions of YAP1 (B), TAZ (C) or TEAD (D) were injected in one-cell stage Tg(Hsa.CTGF:nlsmCherry)ia49 embryos. The fluorescent reporter expression was documented and quantified at 24 hpf. Control morpholino-injected: n = 20; Yap/Taz morpholino-injected: n = 29; controls for YAP-5SA: n = 12; YAP-5SA mRNA-injected: n = 12; controls for TAZ-4SA: n = 57; TAZ-4SA mRNA-injected: n = 56; controls for TEAD-VP16: n = 20; TEAD-VP16 mRNA-injected: n = 20. A.U.: arbitrary units; *p < 0.05; ***p < 0.001. |
|
Analysis of Tg(Hsa.CTGF:nlsmCherry)ia49 reporter activation. (A–A”) In vivo confocal images of Hsa.CTGF:nlsmCherry fluorescence at 20 hpf. (B) Confocal sagittal section of the eye at 24 hpf. (C) Lens of a Tg(Hsa.CTGF:nlsmCherry)ia49 adult fish. (D) Confocal Z-stack projection at 48 hpf showing the transgene expression in the rhombencephalon (r) and the midbrain-hindbrain boundary (MHB) regions. (E) Confocal Z-stack projection of the trunk at 48 hpf. (F–G) Sections of the Z-stack projection in (E) are shown to highlight reporter activation in the somatic muscle (F), in the floorplate, the neural tube and the notochord (G). A strong signal is detected in a row of nuclei located between the neural tube and the notochord and representing the floorplate. The neural tube is only weakly positive. Below the floorplate, the notochord is expressing mCherry in the nuclei located just dorsally and ventrally with respect to the dark stripe of the notochord cells vacuoles. (H) Reporter fluorescence is visible in the epidermis along the whole embryo; here in a confocal Z-stack projection of the terminal portion of the tail at 48 hpf. (I–J) Confocal Z-stack projections of the rostral region of a 72 hpf reporter embryo. In the inset, a dorsal view highlighting the mCherry expression in the pectoral fins is depicted. (K) Confocal sagittal section of the heart region of a Tg(Hsa.CTGF:nlsmCherry)ia49/Tg(myl7:GFP) double transgenic embryo at 22 hpf. (K’–K”’) Inset of K: mCherry channel (K’), GFP channel (K”), merge (K”’). (L) Confocal sagittal section of the heart at 48 hpf. (L’–L”’) Inset of L: mCherry channel (L’), GFP channel (L”), merge (L”’). (M–M’) Heart of a Tg(Hsa.CTGF:nlsmCherry)ia49 adult fish. (N,O) Confocal Z-stack projections at 6 days post fertilization (dpf), showing the strong transgene expression in the whole intestine. (N’) Inset of N, single confocal sagittal section, bright field and mCherry merge, focusing on the reporter expression in the anterior intestine. (O’) Inset of O, single confocal sagittal section, bright field and mCherry merge, focusing on the reporter expression in the mid-posterior intestine. (P) Confocal sagittal section of the mid-posterior intestine of a 6 dpf Tg(Hsa.CTGF:nlsmCherry)ia49/Tg(gut:GFP)s854 double transgenic larva. (P’) Merge with bright field. r: rhombencephalon; MHB: midbrain-hindbrain boundary; v- ventricle; a: atrium; b.a.: bulbus arteriosus; s.b.:swim bladder; a.i.: anterior intestine; p.i.: posterior intestine. Scale bar: 100 μm. EXPRESSION / LABELING:
|
|
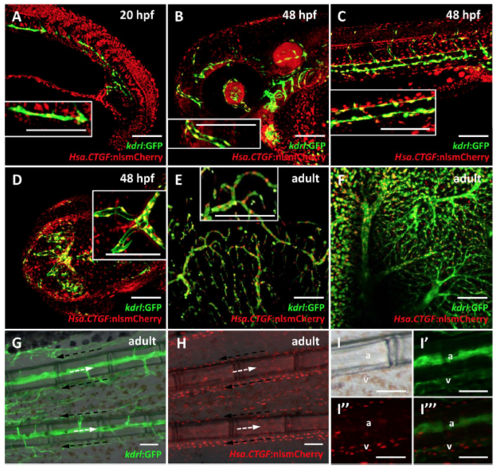
Tg(Hsa.CTGF:nlsmCherry)ia49 reporter activity is prominent in the endothelium. (A–D) Reporter expression in the endothelium during development from 20 to 48 hpf. (E–I) Reporter expression in the adult endothelium. (A) Confocal sagittal section of the trunk of a 20 hpf Tg(Hsa.CTGF:nlsmCherry)ia49/Tg(kdrl:GFP) double transgenic embryo. Tg(kdrl:GFP) expresses GFP in all endothelial cells. Yap1/Taz are active in the endothelial cells in the developing vessels, as shown by the co-localization between the two signals. (B–D) Confocal Z-stack projections of the head region (side view in (B), dorsal view in (D)) and the trunk (C) at 48 hpf. Reporter signal co-localizes with kdrl:GFP expression throughout the embryo. (E,F) Confocal Z-stack projection of brain (E) and liver (F) tissue of a double transgenic adult fish, displaying Yap1/Taz reporter activation in the endothelium of respectively the cerebral and hepatic vascular networks. The insets represent zoomed views highlighting the co-localization between Hsa.CTGF:nlsmCherry and kdrl:GFP. (G–I) Fluorescent microscope images of adult caudal fin in Tg(kdrl:GFP) (G) and Tg(Hsa.CTGF:nlsmCherry)ia49 (H). Lateral views, anterior to the left, dorsal to the top. Arterial (white arrows) and venous (black arrows) bloodstream is indicated. Yap1/Taz reporter activity is stronger in the veins running laterally to the bony fin rays with respect to arteries inside the bony rays, as highlighted in the single channels and merge magnifications (I–I”’). a: artery; v-vein. Scale bar: 100 μm. EXPRESSION / LABELING:
|
|
Yap1/Taz are required for PCV development. (A) Gross morphology of 20 hpf WT and yap1−/−;taz+/− embryos. Red arrows point to the undulating notochord. (A’) Gross morphology of 32 hpf WT and yap1−/−;taz+/− embryos. (B,B’) Transverse sections of the trunk revealing the relative positions of the neural tube (NT), dorsal aorta (DA) and posterior cardinal vein (PCV). The PCV of yap1−/−;taz+/− animals has deviated from the midline and appears to split into two lumenized vessels (arrows). White signal, etv2:EGFP transgene expression; red signal, lyve1b:DsRed transgene expression. The PVC lumen is marked with an asterisk (*), while the DA lumen is marked with a yellow “ + ”. (C,D) Analysis of the deviation of the PCV from the midline in 48 hpf embryos. The data are obtained from a series of transverse sections starting from the caudal end of the yolk tube and moving 50 sections rostrally. The NT, DA and PCV were manually demarcated and the deviation of the PCV from the midline was defined as β = 180°-ϴ. With ϴ (see panel B) we defined the angle formed by the NT, DA, and PCV in each transverse section. Each value of β was plotted for WT and yap1−/−;taz+/− trunks at 48 hpf. Each line represents a single animal. 4/6 of yap1−/−;taz+/− embryos exhibited a PCV that appears to split into two lumenized vessels that are lyve1b:DsRed positive (marked with a circle on the graph). Red horizontal lines above and below 0° are the WT maxima and minima. P values were calculated by the F-test, which tests whether the spread of angles (180° - ϴ) between WT and mutants is the same. σmut2: variance of (180° - ϴ) in yap1−/−;taz+/− animals; σWT2: variance of (180° - ϴ) in WT animals. (E) Whole mount in situ hybridization (WISH) for the expression of efnb2a and mrc1a, markers of arteries and veins, respectively. Red arrows point to expression of mrc1a in the region of the DA. The fraction of the embryos exhibiting the phenotype shown in each image was reported in the upper right corner of the corresponding panel. Scale bars, 100 μm. PHENOTYPE:
|
|
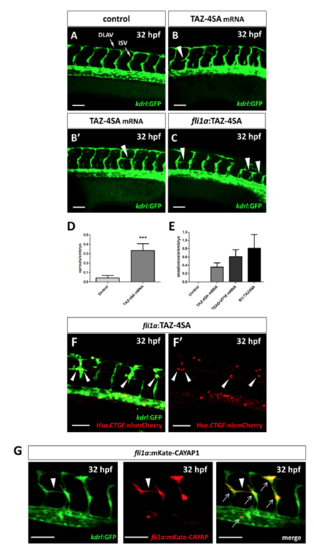
Yap1/Taz activity upregulation promotes vessel sprouting. (A–C) Confocal Z-stack projections of the midtrunk region of Tg(kdrl:GFP) 32 hpf embryos. (A) Representative image of a control injected embryo. (B,B’) Two TAZ-4SA mRNA injected embryos, showing an aberrant ISV sprout (arrowhead in B) and an anastomosis between adjacent ISVs (arrowhead in B’). (C) A mosaic embryo injected with pDestTol2CG2-fli1a-TAZ-4SA plasmid, showing three anastomosis between adjacent ISVs (arrowheads in C). (D,E) Quantification of the aberrant sprouting caused by Yap1/Taz activity upregulation. The number of non-anastomosed aberrant sprouts (D) and the number of anastomosis (E) between adjacent ISVs were evaluated. Both phenomena, observed in TAZ-4SA mRNA and pDestTol2CG2-fli1a-TAZ-4SA mosaic injected embryos, are extremely rare or absent at all in the controls. Controls: n = 49; TAZ-4SA mRNA-injected: n = 42; TEAD-VP16 mRNA-injected: n = 31; pDestTol2CG2-fli1a-TAZ-4SA plasmid-injected: n = 16. (F,F’) Tg(Hsa.CTGF:nlsmCherry)ia49/Tg(kdrl:GFP) double transgenic embryos injected with the pDestTol2CG2-fli1a:TAZ-4SA vector. A strong overactivation of the Hsa.CTGF reporter signal was observed in the nuclei of the endothelial cells undergoing anomalous sprouting (arrowheads in F) with respect to the other normal ISVs. (G) In mosaic embryos injected with the PCS2-fli1a:CAYAP-mKate plasmid a specific expression of the mKate was reported in conjunction with the anomalous endothelial sprouts (arrowhead). The plasmid is endothelium-specific, as highlighted by the co-localization (arrows) between the mosaic mKate and the GFP of the stable transgenic line Tg(kdrl:EGFP). Lateral view, anterior to the left, dorsal to the top. ***p < 0.001. ISV: intersegmental vessel; DLAV: dorsal longitudinal anastomotic vessel. Scale bar: 50 µm. |
|
Overview of the Tg(Hsa.CTGF:eGFP) reporter expression. In vivo fluorescent microscope (A-E’) and confocal images (F-G’) of Tg(Hsa.CTGF:eGFP) larvae at 24, 48 and 72 hpf. |
|
Hsa.CTGF-based reporter signal is dependent on Yap1/Taz in specific tissues. (A,A’) Confocal Z-stack projections of the eye of Tg(Hsa.CTGF:nlsmCherry)ia49 embryos injected with Yap1/Taz or control morpholinos. Yap1/Taz knockdown considerably reduces the reporter signal in the eye. (B,B’) Confocal Z-stack projections of the trunk of Tg(Hsa.CTGF:eGFP)ia48 embryos injected with Yap1/Taz or control morpholinos. The decrease of the reporter signal upon Yap1/Taz knockdown is clearly visible in the floorplate and the PCV. (C-D’’) Confocal Z-stack projections of the heart region of Tg(Hsa.CTGF:nlsmCherry)ia49/Tg(2xID1BRE:GFP)ia17 embryos injected with Yap1/Taz or control morpholinos. Tg(2xID1BRE:GFP)ia17 is used here to highlight the heart structure. Yap1/Taz knockdown reduces the Yap1/Taz reporter signal in the heart. PCV: posterior cardinal vein. |
|
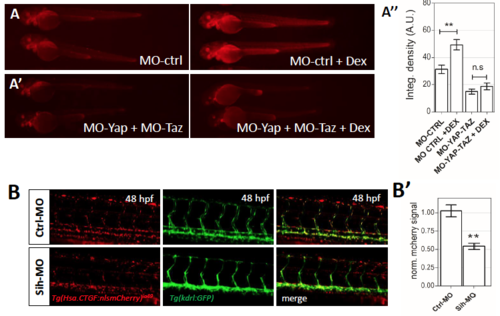
Hsa.CTGF-based Yap1/Taz zebrafish reporter is modulated by glucocorticoids and blood flow. (A,A’) Representative image of Tg(Hsa.CTGF:nlsmCherry)ia49 transgenic embryos injected with Yap1/Taz or control morpholinos alone or combined with 25 μM Dexamethasone (DEX) from 24 hpf to 48 hpf. Dex-activation of Hsa.CTGF transgene is maintained in control morpholino-injected embryos and ablated in Yap/Taz morphants. (A’’) Average values of fluorescence integrated density calculated for treated embryos and controls. For each group a minimum of 10 embryos were analyzed. (B) Representative confocal images of Tg(Hsa.CTGF:nlsmCherry)ia49/tg(kdrl:GFP) double transgenic embryos injected with control or sih morpholinos at 48 hpf. In sih morphants, the activity of the hCTGF reporter in the endothelium is reduced. (B’) Average values of fluorescence integrated density calculated for treated embryos (n=5) and controls (n=6). The mCherry fluorescence was measured in the endothelial cells and normalized for the GFP fluorescence used as internal standard. A.U., arbitrary units. ** = p<0.01. |
|
Yap1/Taz reporter expression is activated maternally. (A-A’’) 50%-epiboly stage embryos from Tg(Hsa.CTGF:nlsmCherry)ia49 female showing the ubiquitous maternally activated Yap1/Taz reporter fluorescence. Brightfield (A), fluorescence (A’) and confocal (lateral view) (A’’) images are shown. (B-B’) Internal organs of a Tg(Hsa.CTGF:nlsmCherry)ia49 adult female. A strong reporter fluorescence is detected especially in the ovary. (C-C’) Zoomed view of the ovary. Transgene signal is visible in the nuclei (n) of the oocytes at advanced maturation stages and in the accompanying follicle cells, arranged in a single layer adjacent to the oocytes (arrowheads). Scale bar: 100 μm. EXPRESSION / LABELING:
|
|
Time series of Yap1/Taz reporter expression during embryonic and early larval stage. Confocal Z-stack projections of a Tg(Hsa.CTGF:nlsmCherry)ia49 fish at 19 hpf (A-B’’), 24 hpf (C-D’’), 48 hpf (E-F’’), 72 hpf (G-H), and 6 dpf (I-L). (A, C, E, G, I) Full zstack projection of the rostral region of the embryo/larva. (B, D, F, H, L) Full z-stack projection of the trunk of the embryo/larva. Partial z-stack projection limited to the optic cup (A’, C’, E’), the rhombencephalon (A’’, C’’, E’’), the somitic muscles (B’, D’, F’), and the notochord region (B’’, D’’, F’’) are reported in the magnified views. Relevant cell types where the reporter is strongly activated are highlighted at each time point. For the details regarding the reporter protein expression in the different tissues refer to the text and to Figures 3 and 4. |
|
CRISPR targeted mutagenesis of the zebrafish yap1 and taz loci. Single guide RNAs (sgRNAs) were designed to target the first exon of yap1 and the second exon of taz. Sequence alignments between WT and mutant sequences for yap1bns19 (A) and tazbns35 (B). The underlined nucleotides are the PAM sequences. (A’ and B’) These indel mutations result in a frameshift and are predicted to encode a truncated protein product as represented in the cartoon. (A’’ and B’’) The genotyping of these alleles can be performed by simple PCR followed by resolution of the WT and mutant amplicons by gel electrophoresis. (C) Representative confocal images of Tg(Hsa.CTGF:nlsmCherry);TgBAC(etv2:EGFP) transgenic embryos in yap and taz mutant background. Higher magnification images correspond to the region demarcated in yellow. (C’) The mCherry fluorescence was measured in the endothelial cells and normalized for the GFP fluorescence used as internal standard. n for each group is indicated. * = p<0.05. |
|
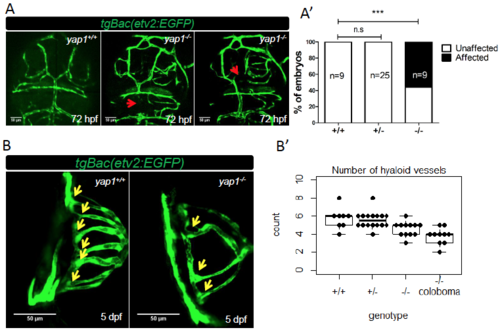
yap1-/- larvae exhibit cranial and hyaloid vasculature defects. (A) Representative figures of yap1 mutants showing cranial vasculature defects compared to WT sibling at 72 hpf. The red arrows point to lack of endothelial transgene expression in the mesencephalic vein (MsV) and dorsal longitudinal vein. (A') The cranial vasculature phenotype is partially penetrant. (B) Dorsal view of the eyes of 5 dpf WT and mutant siblings. (B') Number of hyaloid vessels per embryo for each genotype. Each point represents one eye. The number of hyaloid vessels of wild-type embryos is statistically higher in comparison to mutants with (p<0.01) or without (p<0.05) coloboma by student T test. n for each group is indicated. *** = p<0.001. PHENOTYPE:
|
|
PCV defects in 30 hpf yap1-/-;taz+/- embryos. (A) Transverse sections of trunk to show the relative positions of the neural tube (NT), dorsal aorta (DA) and posterior cardinal vein (PCV) in tgBac(etv2:EGFP). (B, C) Maximum intensity projections from the right side. (B’, C’) Quantification of PCV deviation was performed as described in Figure 5B-D. σmut 2: variance of (180° - ϴ) in yap1-/-;taz+/- animals; σWT 2: variance of (180° - ϴ) in WT animals. n for each group is indicated. |
|
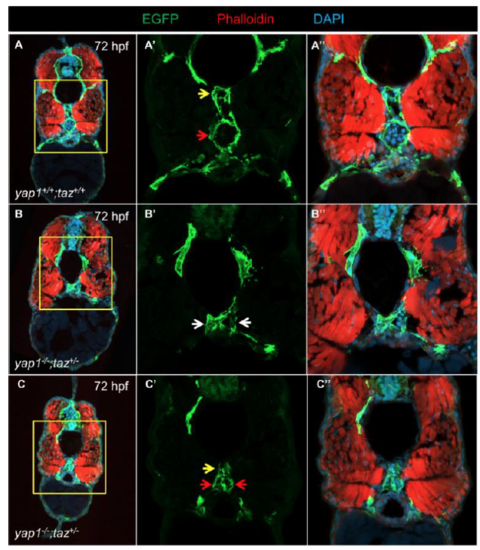
PCV defects in 72 hpf yap1-/-;taz+/- animals. Transverse cryosection of TgBac(etv2:EGFP) WT (A) and yap1-/-;taz+/- (B and C) larvae at 72 hpf. (A’-C’, A’’-C’’) High magnification images of regions demarcated in yellow. Yellow arrows: DA; red arrows: PCV; white arrows: DA or PCV. |