- Title
-
Cxcl12a induces snail1b expression to initiate collective migration and sequential Fgf-dependent neuromast formation in the zebrafish posterior lateral line primordium.
- Authors
- Neelathi, U.M., Dalle Nogare, D., Chitnis, A.B.
- Source
- Full text @ Development
|
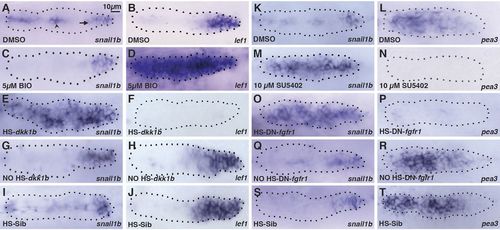
Wnt-dependent Fgf signaling inhibits snail1b expression in the trailing domain. (A,B) Expression of snail1b and Wnt-dependent lef1 in leading domain of pLL primordium in control DMSO-treated embryos at 32 hpf. Arrow and arrowhead show snail1b in the pLL primordium and in the underlying horizontal myoseptum cells, respectively. (C,D) snail1b and lef1 expression in BIO-treated embryos. (E-J) snail1b and lef1 expression following heat shock in HS:dkk1b embryos (E,F), without heat shock in HS:dkk1b embryos (G,H) and with heat shock in non-transgenic siblings (I,J). (K,L) Expression of snail1b and Fgf-dependent pea3 at 32 hpf in control DMSO-treated embryos. (M,N) snail1b and pea3 expression in SU5402-treated embryos. (O-T) snail1b and pea3 expression following heat shock in HS:DN-fgfr1 embryos (O,P), without heat shock in HS:DN-fgfr1 embryos (Q,R), and with heat shock in non-transgenic siblings (S,T). Fluorescent anti-GFP staining in the Tg[cldnb:lynGFP] line was used to define the outline of all pLL primordia (dotted line). The primordia leading end is always to the right. n=20 for each of three independent experiments. |
|
Cxcl12a promotes expression of snail1b in the leading domain. (A,B) snail1b expression at the leading end of the primordium in the control embryos (A) and loss of snail1b expression following cxcl12a knockdown (B). (C) snail1b expression at both ends (brackets) following cxcr7b knockdown. (D) Broad snail1b expression in heat-shocked HS:cxcl12a transgenic embryos (HS-cxcl12a). (E,F) snail1b expression in non-heat-shocked transgenics (NO HS-cxcl12a) (E) and in heat-shocked non-transgenic siblings (HS-Sib) (F). (G) cxcl12a induction in the embryo following heat shock-induced expression of cxcl12a in HS:cxcl12a transgenic embryos. (H,I) Lack of cxcl12a induction in non-heat-shocked (H) and in heat-shocked non-transgenic siblings (I). n=20 for each of two independent experiments. Dotted lines encircle pLL primordia. |
|
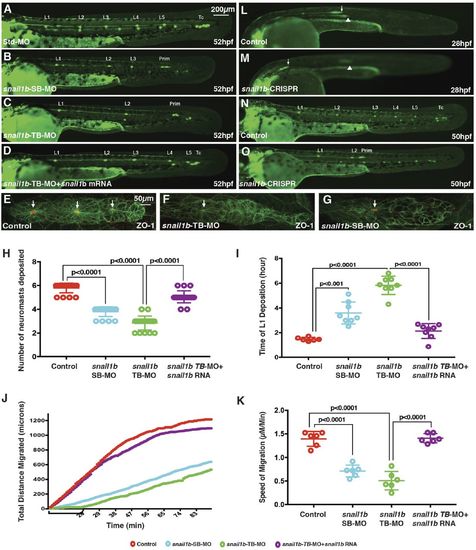
Delayed neuromast formation and primordium migration following reduction of snail1b function. (A-D) The pattern of neuromast deposition visualized in CldnB:lyn-GFP embryos at 52 hpf in control (A), snail1b splice-blocking morpholino (SB-MO)-injected embryos (B), snail1b translation-blocking morpholino (TB-MO)-injected embryos (C) and snail1b-TB morphants co-injected with snail1b mRNA (D). (E-G) Expression of ZO-1 (red) in a control embryo (E), a TB morphant (F) and an SB morphant (G). Arrows indicate ZO-1 at apical constriction of protoneuromasts. (H) Graphs comparing the number of neuromasts deposited (H) and time of first neuromast (L1) deposition (I) in various experimental conditions. (J) Plot comparing the total distance travelled by the primordium with time in control, SB-MO-injected, TB-MO-injected, and TB-MO and snail1b mRNA co-injected embryos. Bracket below the x-axis highlights the delay in initiation of migration in snail1b morphants. (K) Comparison of the average migration speed of the primordium. Significance in H,I and K was determined by Student's unpaired t-test. In H,I,K, error bars represent standard error of the mean. (L,M) Primordium position (arrow) in a control embryo at 28 hpf (L) and in a snail1b-CRISPR-injected embryo (M). Arrow shows primordium on one side with delayed migration, and arrowhead points to slightly out of focus primordium on the contralateral side at a position similar to that in control embryos. (N,O) Pattern of neuromast deposition in a control embryo (N) and an example of a snail1b-CRISPR-injected embryo with fewer neuromasts and incomplete primordium migration (O) at 50 hpf. n=20 for each of three and two independent morpholino and CRISPR experiments, respectively. Prim, primordium; Tc, terminal cluster. |
|
Snail1b is required in the migrating primordium for effective migration and its loss has reciprocal effects on of cdh2 and epcam expression. (A-A″) Stills from a time-lapse movie of pLL primordia migration in snail1b morphant embryo (Movie 3) at 0 h (A), 2 h (A′) and 6 h (A″). The dorsal view shows the left and right primordia. Transplanted wild-type cells (magenta) are localized in the left primordium and overlying skin but not in underlying horizontal myoseptum. (B-B″) Example of a primordium in a TB-MO-injected Cldn B:lynGFP transgenic embryo with transplanted cells from a non-morphant donor embryo but without transplanted cells in the underlying horizontal myoseptum cells. Transplanted cells are labeled with rhodamine-dextran. (C-J) Expression of cdh2 and epcam in control primordia (C,D), in snail1b-TB morphants (E,F), in snail1b-TB morphants co-injected with snail1b RNA (G,H) and in snail1b mRNA-injected embryos (I,J). (K-N) cdh2 and epcam expression in DMSO- and PP1-treated embryos. (O,P) Quantification of cdh2 and epcam expression following inhibition of migration by the Src inhibitor PP1 and in snail1b morphants. Error bars represent standard error of the mean. (Q-S) Reversal of slower primordium migration defect in snail1b morphants by co-injection of epcam morpholinos. Arrow shows position of the primordium. (T) Primordium migration is similar to controls in embryos injected with epcam-MO alone. (U,V) Ectopic expression of epcam mRNA slows migration of the primordium (prim). (W) Distance migrated by control and epcam mRNA-injected embryos. Error bars represent standard error of the mean. (X-Z) epcam expression expands into the leading zone of slower migrating primordia of snail1b CRISPR-injected embryos. X show a lower magnification of the embryo shown in Y,Z, which show the slower migrating primordium (Prim A) and the contralateral faster migrating primordium (Prim B). The primordium with delayed migration (Y) has expanded epcam expression in the leading domain compared with the contralateral side (Z), where there is a lower level of expression in the leading domain. n=13 for transplants, n=25 for each of two independent experiments for in situ hybridization. n=20 and 14 for each of two independent experiments of epcam rescue and overexpression, respectively. Dotted lines encircle pLL primordia. |
|
Delayed Wnt Fgf signaling dynamics in the primordium of snail1b morphants. (A-C) Progressive restriction and eventual loss of lef1 expression in controls from 26 to 48 hpf. (D-F) Delayed restriction and persistent lef1 expression in snail1b morphants. (G-I) pea3 expression from 26 to 48 hpf in control embryos. (J-L) Delayed establishment and persistent trailing expression of pea3 expression in snail1b morphants. (M-O) cxcr4b expression from 26 to 48 hpf in control embryos. (P-R) Broad persistent cxcr4b expression in snail1b morphants. (S-U) cxcr7b expression from 26 to 48 hpf in control embryos. (V-X) Delayed establishment and persistent cxcr7b expression in snail1b morphants. (Y) Graph of length of the lef1 expression domain in control and snail1b-MO embryos plotted against the distance migrated by the pLL primordium. n=25 for each of two independent experiments if not stated in the text, n=147 and 144 for control and snail1b-MO for the data shown in Y. Dotted lines encircle pLL primordia. |
|
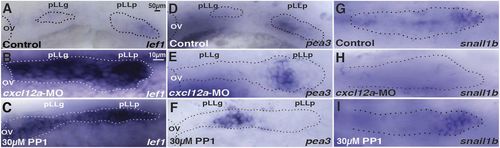
Delayed Wnt Fgf signaling dynamics in stalled primordia. (A) In control embryos, the primordium (pLLp) migrates and separates from the pLL ganglion (pLLg) by 22 hpf and lef1 expression is restricted to the leading domain. (B,C) With migration inhibited in cxcl12a morphants and PP1-treated embryos, the primordium remains juxtaposed with the pLLg and lef1 expression remains broad. (D) pea3 expression at the trailing end of the primordium in controls at 22 hpf. (E,F) The pea3 expression domain is still at the trailing end of the prospective primordium in the absence of migration; however, the pea3 domain is in the middle of a broad lef1-expressing domain, which now represents the juxtaposed primordium and the pLLg. (G-I) snail1b expression in control, cxcl12a morphant and PP1-treated primordia at 22 hpf. OV, otic vesicle. n=25 for each of two independent experiments. Dotted lines encircle pLL primordia. EXPRESSION / LABELING:
PHENOTYPE:
|
|
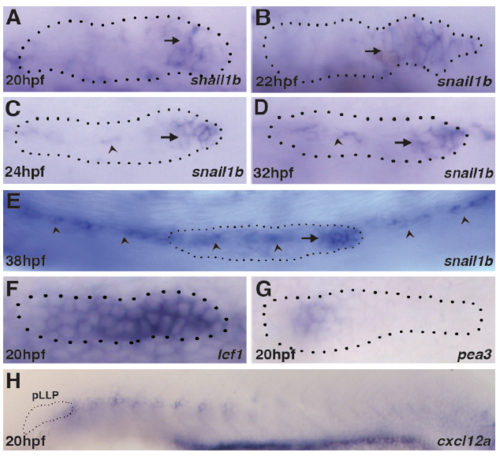
snail1b expression in the leading domain (arrow) at (A) 20hpf, (B) 22hpf, (C) 24hpf (D) 32hpf and (E) 38hpf. arrow heads in C-E show snail1b expression in the underlying cells of the horizontal myospetum. (F) At 20hpf, Wnt-dependent lef1 expression is broad and, (G) FGFdependent pea3 has not been effectively established. (H) However, at 20hpf the primordium is only exposed to cxcl12a
|
|
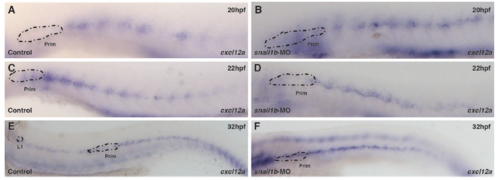
The delay in initiation of primordium migration in snail1b morphants is not due to aberrant cxcl12a expression by underlying snail1b-expressing horizontal myoseptum cells. cxcl12a begins to express on the horizontal myospetum at 20hpf (A) and it forms a stipe over the entire embryo by 22hpf (C) and continues to express at 32hpf (E) in controls. snail1b morphants have similar spatiotemporal pattern of cxcl12a expression (B,D,F). |
|
Validation of epcam-MO. (A-C) Injection of 1.2ng epcam morpholino recapitulated a previously published epcam mutant phenotype. It prevented normal morphogenesis of otoliths (shiny crystals lost- arrows). Co-injection of 30ng rescued this morphant phenotype. (D-G) As in the epcam mutants, there was no obvious lateral line phenotype with injection of 1.2ng epcam morpholino. (G) However, when the dose of epcam morpholino was increased to 5 ng, a more variable number of neuromasts was deposited, consistent with previous studies that had suggested that the epcam morpholinos can have some off-target effects. For our study only the low dose of 1.2 ng was used to minimize potential off target effects. Fig. |
|
Rescue of the delayed dynamics of Wnt Fgf signaling systems in snail1b morphants by coinjection of snail1b mRNA. Expression of lef1 (A), pea3 (E), cxcr4b (I) and cxcr7b (M). lef1 (B) and cxcr4b (J) are expanded into the trailing domain (B), pea3 (F) and cxcr7b (N) are not well established in the trailing domain of snail1b-MO. Injection of snail1b mRNA along with snail1b- TB MO restores the expression patterns of lef1 (C), pea3 (G), and cxcr7b (O). However, restricted cxcr4b expression is not restored (K). Injected of snail1b mRNA alone does not have any obvious effect on these signaling systems (D, H, L, P). |