- Title
-
Direct activation of chordoblasts by retinoic acid is required for segmented centra mineralization during zebrafish spine development
- Authors
- Pogoda, H.M., Riedl-Quinkertz, I., Löhr, H., Waxman, J.S., Dale, R.M., Topczewski, J., Schulte-Merker, S., Hammerschmidt, M.
- Source
- Full text @ Development
|
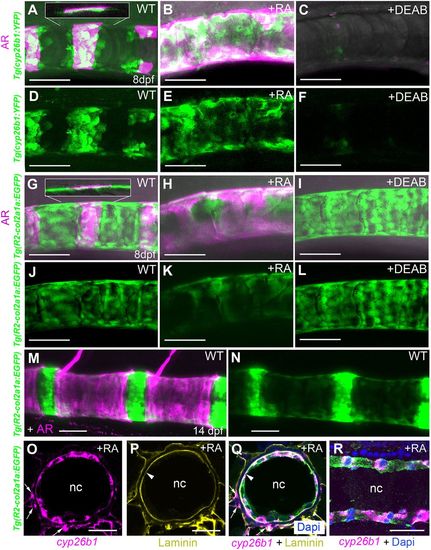
Centra-associated subsets of otherwise uniformly distributed chordoblasts express cyp26b1 in an RA-dependent manner. (A-N) Lateral views of notochords at the level of centra 3-5 in control (A,D, n=67; G,J, n=72; M,N, n=10), RA-treated (B,E, n=84/84; H,K, n=53/53) or DEAB-treated (C,F, n=77/77; I,L, n=32/32) larvae bearing the indicated YFP- or GFP-encoding transgenes. Anterior is to the left; calcified extracellular matrix is labeled by Alizarin Red. (A-C,G-I,M) Projections of confocal stacks with merged green, magenta and transmitted light channels. (D-F,J-L,N) Corresponding single green channel projections. Insets (A,G) show single confocal planes, revealing that YFP- and GFP-positive cells are located on the inner surface of the mineralized notochord sheath. (O-R) Fluorescence in situ hybridization of cyp26b1 transcripts on transverse (O-Q; level of chordacentra) and sagittal (R) sections through the notochord, co-labeled by immunofluorescence against Laminin (yellow) and Tg(R2-col2a1a:EGFP)-encoded EGFP (green). Arrowheads highlight notochord basement membrane, arrows cyp26b1-positive cells outside the notochord, possibly representing somite-derived scleroblasts generating the outer autocentra (Bensimon-Brito et al., 2012). Scale bars: 50 µm (A-N); 20 µm (O-R). AR, Alizarin Red; nc, notochord; WT, DMSO/ethanol-treated wild-type control. |
|
cyp26b1-positive chordoblasts co-express the biomineralization gene entpd5a in an RA-dependent manner. (A-I) In situ hybridization for entpd5a transcripts (magenta) on transverse (A,B,D,E; level of chordacentra) or sagittal (C,F,G-I) notochord sections of wild-type (A-F), control- (G), RA- (H) or DEAB-treated (I) larvae at 7 dpf, co-labeled with DAPI (blue), anti-Laminin (yellow), anti-Tg(R2-col2a1a:EGFP)-derived EGFP (green) and/or anti-Tg(cyp26b1:YFP)-derived YFP immunofluorescence (green). (D-F) Magnifications of the areas demarcated by brackets in A-C. Upper panels of D-F show cognate images of merged channels, middle and lower panels single channel images for entpd5a expression (magenta) and anti-GFP or anti-YFP immunofluorescence (green), respectively. Scale bars: 20 µm (A,B,D,E); 50 µm (C,F,G-I). Arrowheads (C,G,H) indicate somite borders. (J) qRT-PCR analysis of entpd5a transcript levels in trunks of 7 dpf larvae after 3 days of RA or DEAB treatment (n=30 per condition); mean±s.d.; ***P<0.0001. (K) qRT-PCR analysis of cyp26b1 and entpd5a transcript levels in trunks of 5 dpf larvae after 1 h of RA treatment (n=20 per condition); mean±s.d.; ***P<0.0001. (L) Schematic model of the molecular events at a forming chordacentrum: RA, generated and regulated by thus far unknown cells and upstream factors, co-induces chordoblast-specific expression of entpd5a and cyp26b1. Cyp26b1 enzyme inactivates RA at its site of action to restrict lateral spreading of the signal and to ensure physiologically appropriate RA concentrations. nc, notochord. |
|
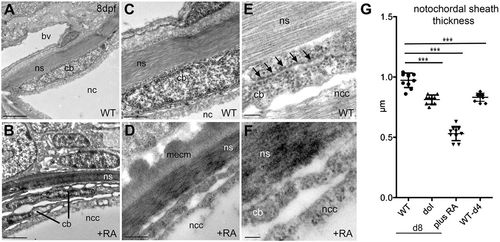
Chordoblasts of RA-treated zebrafish larvae acquire preosteocyte-like morphological features. (A-F) Transmission electron micrographs of transverse sections through the notochord of control- (A,C,E; level of prospective intervertebral spaces) and RA-treated (B,D,F) larvae at 8 dpf. Scale bars: 2 µm (A,B); 0.5 µm (C,D); 0.1 µm (E,F). Note the higher electron density of the notochord sheath in B and D, indicative of its strong mineralization. bv, blood vessel; cb, chordoblast; mecm, mineralized extracellular matrix; nc, notochord; ncc, notochordal cell; ns, notochordal sheath. Arrows in E point at a stretch of rough endoplasmic reticulum, which is barely found in chordoblasts of RA-treated larvae (F). (G) Quantification of notochord sheath thickness in different genetic backgrounds or after RA treatment as indicated; n=10 per condition; mean±s.d.; ***P<0.0001. dol, cyp26b1ti230g mutant (dolphin) (Laue et al., 2008). At 8 dpf (d8), the mutant shows reduced thickness of the notochord sheath, comparable to the thickness of wild types at 4 dpf (d4), most likely due to the premature reduction of sheath formation in chordoblasts. In wild-type larvae treated with RA from 4 to 8 dpf, the width of the sheath is even more strongly reduced, possibly pointing to additional RA-induced active sheath resorption (compare with Jeradi and Hammerschmidt, 2016; To et al., 2012). |
|
Biomineralization of the notochord sheath is impaired upon chordoblast-specific cell ablation or RA reception inhibition. (A-D) Lateral views of notochords at the level of centra 3-5 in control-treated (A, n=42), RA-treated (B, n=25), Mtz-treated (C, n=63/63) and RA+Mtz-treated (D, n=47/47) R2-col2a1a:EGFP; col2a1a:CFP-NTR double transgenics at 8 dpf; confocal projections with merged green (GFP), magenta (Alizarin Red), blue (CFP-NTR) and transmitted light channels; panels A′ and C′ show single channels of middle parts of A and C, respectively. (E-G) Transmission electron micrographs of transverse sections through the notochord sheath at the level of chordacentra of untreated larvae at 4 dpf (E, n=5) or 8 dpf (G, n=5), and of Mtz-treated R2-col2a1a:EGFP; col2a1a:CFP-NTR double-transgenic at 8 dpf (F, n=6). The final width of the notochord sheath has almost been reached at 4 dpf and is not significantly affected by chordoblast ablation via Mtz administration between 4 and 6.5 dpf (compare F and G). (H-K,M) Lateral views of notochords from larvae with the indicated genotypes at 7.5 dpf, levels of centra 1-6, anterior to the left. (H-J) Projections of confocal stacks with merged magenta (Alizarin Red), green (transgene-encoded EGFP-dnRARa) and transmitted light (H, n=25; I, n=14/14; J, n=45/47) channels. Asterisk in I indicates region devoid of EGFP-dnRARa-positive cells that corresponds to centrum #5 in wild type (asterisk in H). Inset in J shows a magnified view of a portion of the notochord with gained intensity of the green channel, revealing nuclear EGFP-Hs-dnRARa fusion protein. (K,M) Stacks of merged yellow (transgene-encoded YFP) and transmitted light (K, n=38; M, n=42/42) channels. (L,N) Immunofluorescence staining on transverse sections through notochords at level of chordacentra from specimens with genotypes as in K and M and with antigen labeling as indicated. Scale bars: 0.5 µm (E-G); 50 µm (A-D,H-K,M); 20 µm (L,N). Arrows in L and N point to chordoblast layer, arrowheads to labeled cells outside the notochord [WT, n=6; Tg(col2a1a:EGFP-dnRAR), n=4; ratio of Tg(cyp26b1:YFP)-positive cells inside and outside the notochord: 26/10 (WT), 3/16 (Tg)]. AR, Alizarin Red; nc, notochord; ns, notochord sheath. |
|
Lineage tracing reveals that centra-associated col2a1- and cyp26b1-positive chordoblasts derive from the early notochord anlage. (A-C) Characterization of the gsc driver used for the axial mesoderm lineage tracing experiments. Dorsal view of a Tg(gsc:Cre); Tg(hsp70:loxp-dsRed-loxpegfp) double transgenic embryo at 15-somite stage (20 hours post fertilization; hpf), 2 hours post heatshock; confocal projection with merged red and green channels. (B) and (C) show the corresponding images of the single channels, respectively. Most notochordal cells express EGFP, whereas no EGFPpositive cells are present outside this structure, indicating the axial mesoderm specificity of the used gsc driver. In particular, sclerotomal cells of the somites are not labelled by the gsc driver. At the shown segmentation stage, notochordal cells have not segregated into outer chordoblasts and inner vacuolated cells as yet. Please note that compared to the endogenous gsc gene, the used gsc driver appears to lack negative cis-regulatory elements that usually repress expression within the notochord anlage, restricting expression to the prechordal plate (Doitsidou et al., 2002). (D) Lateral view of the notochord of a 6 dpf Tg(R2-col2a1a:EGFP); Tg(bact2:loxP-AmCyanloxP-mCherry) double transgenic larva injected with a gsc:Cre construct; confocal projection of merged green, red and transmitted light channels. Mosaic recombination events within the axial mesoderm result in mCherry labeling of some but not all cells deriving from the notochord anlage. The arrowhead points at an mCherry-expressing chordoblast identified as such by co-expression of the R2-col2a1a:EGFP transgene. (E-G) Magnification of the domain harboring the marked chordoblast from (D) in single and merged channels as indicated. In total, 28 chordoblasts positive for the gsc lineage tracer were observed in eight specimens. (H-M) Lateral view of the notochord of a 8 dpf Tg(cyp26b1:YFP); Tg(bact2:loxPAmCyan-loxPmCherry) double transgenic larva injected with the same gsc:cre construct; confocal projection of merged green and red (here magenta) channels (H,K) or the corresponding single channels (I,J,L,M); for better visualization, panels (K-M) show single confocal sections of the upper third from panels (H-J) with focus on marginal chordoblasts of the dorsal notochord border. Mosaic recombination within the axial mesoderm results in mCherry labeling of some chordoblasts as well as vacuolated notochordal cells (ncc). Some of the lineage-traced chordoblasts also coexpress the cyp26b1:YFP transgene (arrows and arrowheads), confirming their association with the centra inducing chordoblast subpopulation (seen in 11 centra of 4 specimens). mCherrypositive but YFP-negative chordoblasts most likely lie outside the RA target domain of the intercentral spaces. (N,O) Lateral view of a notochord at the level of a calcein-stained centrum (green) in a Tg(gsc:Cre); Tg(bact2:loxPAmCyan- loxP mCherry) double transgenic larva at 8dpf, confocal projection of merged red (here magenta) and green channels. Panel (O) shows the lower third of (N) in the single red (magenta) channel visualizing the continuous layer of notochord anlagen derived, mCherry-positive chordoblasts. Some of these cells are positioned directly underneath the mineralized matrix of the chordacentrum. Scale bars correspond to 20μm in (D-O) and to 50μm in (AC). |
|
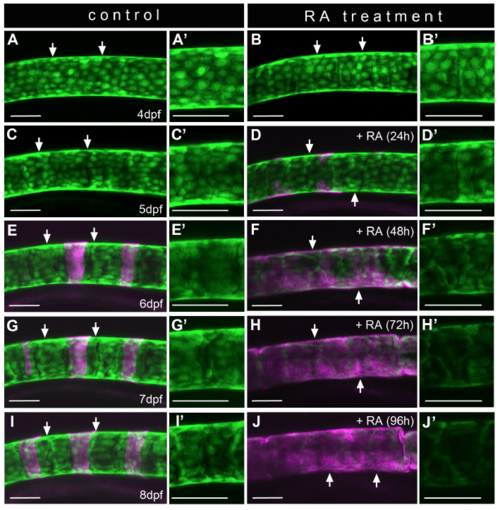
Down-regulation of Tg(R2-col2a1a:EGFP) expression and alterations in chordoblast morphology upon RA treatment are progressive processes (A-J) Lateral views of notochords at the level of centra #2 to #4 in a representative control- (A,C,E,G,I; n=8) or RA-treated (B,D,F,H,J; n=12) Tg(R2-col2a1a:EGFP) larva in a time series over the indicated ages, anterior to the left; calcified extracellular matrix is labeled via Alizarin red (magenta). Time of RA incubation in the treated specimen (right column) is shown in brackets. Panels (A-J) show projections of confocal stacks with merged green and magenta channels. Panels (A’-J’) show single green channel projections of the corresponding area framed by the two arrows in (A-J). Scale bars represent 50μm. Note that the decrease in transgene expression is accompanied by a gradual change in chordoblast morphology, and that both processes occur in a progressive manner, with maximal effects only seen after three days of RA treatment. |
|
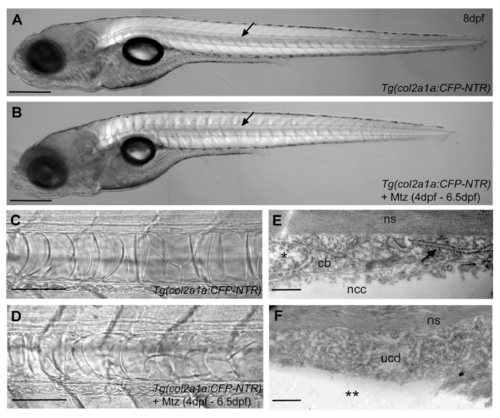
Ablation of chordoblasts does not impair overall notochord integrity. (A,B) Lateral view of an untreated (A; n=42) and a Mtz treated Tg(col2a1a:CFP-NTR) transgenic larva (B; n=63) at 8dpf, anterior is to the left. Even though the chordoblast ablated animal is a bit shorter than the untreated control, it has an obvious notochord (arrow) and an infladed swim bladder, indicating that it does not suffer from retarded development. Panels (C,D) depict magnifications of a stretch of notochord of the same specimens as in (A,B). As in the untreated larva (C), vacuolated notochordal cells persist after chordoblast ablation upon Mtz incubation from 4dpf to 6.5dpf (D). (E,F) TEM micrographs of transverse sections at the level of chordacentra through the notochordal sheath and adjacent chordoblast layer in an untreated control Tg(col2a1a:CFP-NTR) (D; n=5) and an Mtz-treated specimen of the same genotype (F; n=6) at 8dpf. The shown chordoblast (cb) of the untreated control (E) contains an intact nucleus (*) and clearly recognizable rER (arrow), and is directly attached to a proximally positioned notochordal cell (ncc). In the chordoblast-ablated larva (F), no distinct chordoblasts can be found; rather material without any obvious cellular structures designated as “undefined cellular debris” (ucd) can be detected at cognate positions. Underscoring the acellular nature of this material, no directly attached notochordal cells can be observed (**). It is most likely this ucd that gives rise to the remaining rather weak uniform fluorescence seen around the notochord of chordoblast-ablated larvae (compare with Fig.4C,D). Scale bars correspond to 500μm in (A,B), 100μm in (C,D) and 0.5μm in (E,F). Other abbreviations: ns, notochord sheath. |
|
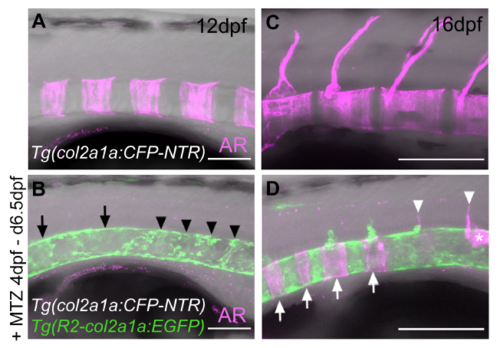
Chordoblast-ablated zebrafish larvae show partial and severely delayed recovery of centra formation. Lateral view of a representative untreated Tg(col2a1a:CFP-NTR) larva (A,C; n=32) and an Mtz-treated Tg(col2a1a:CFP-NTR); Tg(R2-col2a1a:EGFP) double transgenic larva (B,D; n=18) at 12 dpf (A,B) and 16 dpf (C,D; same individuals as at 12 dpf), anterior is to the left. Mineralized matrix is stained via Alizarin red (magenta). All panels represent confocal projections of merged red (here shown in magenta), green and transmitted light channels. Compared to control larvae (A) that show robust iteratively formed centra at 12 dpf none of such structures can be found in siblings of the same age in which chordoblasts have been ablated in a time window between 4 and 6.5 dpf (C). The col2a1a:EGFP transgene in the treated larvae indicates that a few chordoblasts have already recovered in some domains of the notochord (arrows) while other regions are still devoid of distinct chordoblasts (arrowheads). (B) Four days later (16 dpf), the untreated zebrafish larva has massively gained in growth and thus size of the centra, which in the meantime have formed neural arches in most rostral segments. (D) By now, also a rather regular sequence of centra has been formed in the Mtz treated specimen at those positions along the notochord where proper chordoblasts did recover earlier (white arrows). Additionally, mineralized neural arch anlagen (white arrowheads) and some associated mineralized matrix (white asterisks) can be found in adjacent caudal segments where the integrity of the chordoblast layer is still not completely given as indicated by the less intense and irregular chordoblast derived EGFP signal. Further histological analyses would have to reveal whether those mineralized matrices are of chorda- or autocentra nature. Despite this partial axial recovery, treated larvae rarely survive beyond 20dpf, possibly due to malformations of the mandibular apparatus that also expressed the col2a1a:CFP-NTR transgene during Mtz treatment. Scale bars correspond to 100μm in (A,B) and 200μm in (C,D). |