- Title
-
Highly efficient generation of knock-in transgenic medaka by CRISPR/Cas9-mediated genome engineering
- Authors
- Watakabe, I., Hashimoto, H., Kimura, Y., Yokoi, S., Naruse, K., Higashijima, S.I.
- Source
- Full text @ Zoological Lett
|
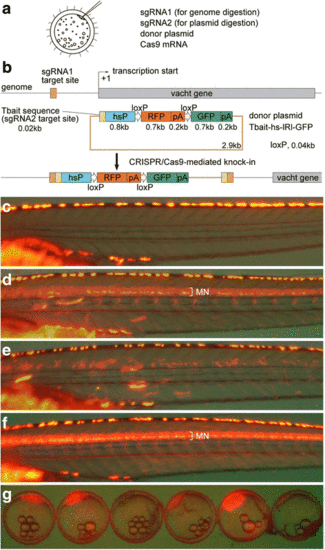
Strategy for the generation of knock-in medaka and generation of Tg[vacht-hs:lRl-GFP] strains. (a) For the generation of knock-in transgenic fish, sgRNA1 (for genome digestion), sgRNA2 (for plasmid digestion), donor plasmid with a bait sequence, and Cas9 mRNA are co-injected into one-cell-stage medaka embryos. (b) A schematic representation of the vacht locus (grey box) and the sgRNA target sites (orange box), and the reporter gene construct consisting of the Tbait (brown box), medaka hsp70 promoter (hsP, blue box), loxP, RFP-pA (red box), loxP, and GFP-pA (green box). After injection, the concurrent cleavage of the targeted genomic locus and the Tbait-hs-lRl-GFP reporter plasmid results in the integration of the reporter by non-homologous end joining (NHEJ). The scheme shows the forward integration of the reporter. (c) Lateral view (red fluorescence) of a control larva at 9dpf. Red-yellow signals in the dorsal region of the body are from the auto-fluorescence of pigment cells. (d) An example of an injected larva. RFP expression was present broadly in the motoneurons (MN). Animals with this kind of RFP expression were judged as having “good expression”, and raised to adulthood. (e) Another example of an injected larva. In this animal, RFP expression was present in the motoneurons, but the number of RFP-expressing cells is much smaller than that in (d). Animals with this kind of RFP expression were judged as not having “good expression”, and were not raised. (f) Lateral view of a Tg[vacht-hs:lRl-GFP] larva. RFP expression was present broadly in the trunk motoneurons. All of the trunk motoneurons are likely to express RFP in this animal. (g) Maternal expression of RFP in early embryos in the Tg[vacht-hs:lRl-GFP] line. The expression levels of RFP are variable among embryos. The embryos were obtained from a single mother |
|
Generation of Tg[zhspa8:Cre-mCherry-NLS] transgenic fish and conversion of RFP transgenic fish to GFP transgenic fish. (a) A schematic of the zhspa8:Cre-mCherry-NLS plasmid. The plasmid consists of the Tol2 left arm (white box), zebrafish hspa8 promoter (zhspa8; blue box), Cre-mCherry-NLS and bovine growth hormone (BGH) polyA signal (red box), and Tol2 right arm (white box). (b) A 1dpf embryo of the Tg[hspa8:Cre-mCherry-NLS] transgenic fish. Red fluorescence derived from Cre-mCherry-NLS is present ubiquitously in the embryonic body. (c) A 9dpf larva derived from the crossing between Tg[vacht-hs:lRl-GFP] and Tg[hspa8:Cre-mCherry-NLS]. GFP instead of RFP is expressed in the trunk motoneurons (MN) |
|
Generation of Tg[nr5a1-hs:lRl-GFP] and Tg[nr5a1-hs:GFP] transgenic fish. (a, b) Dorsal view of the head region (a) and lateral view of the trunk region (b) of a control larva at 9dpf with an RFP filter set. Red signals are from the auto-fluorescence of pigment cells. (c, d) Tg[nr5a1-hs:lRl-GFP] transgenic fish viewed with an RFP filter set. RFP is expressed in cells in the hypothalamus (hp), interrenal gland (ir), and gonad (g). Note that the red signals are not auto-fluorescence, as the corresponding signals are absent in a control larva (a and b). (c', d') Tg[nr5a1-hs:lRl-GFP] transgenic fish viewed with a GFP filter set. No GFP expression is present. (e, f) Tg[nr5a1-hs:GFP] transgenic fish with a GFP filter set. GFP is expressed in cells in the hypothalamus (hp), interrenal gland (ir), and gonad (g). Yellow signals are from the auto-fluorescence of pigment cells. (e', f') Tg[nr5a1-hs:GFP] transgenic fish viewed with an RFP filter set. No RFP expression is present |
|
Generation of Tg[pax7a-hs:GFP] and Tg[sox5-hs:GFP] fish. (a) Schematic of the knock-in strategies. The sgRNAs for pax7a or sox5 were designed to target a sequence upstream of the initiation methionine that corresponds to the 5′ untranslated region. (b–d) Tg[pax7a-hs:GFP]. (e–g) Tg[sox5-hs:GFP]. (b, b', c, c', e, e', f, f') Dorsal views of the head (b, b', e, e') and the trunk (c, c', f, f') at 2 dpf in the bright field (b, c, e, f) and in fluorescence (b', c', e', f'). (d, g) Dorsal (upper) and lateral (lower) views at 9 dpf. In Tg[pax7a-hs:GFP] embryos, GFP is expressed in the tectum, hindbrain, anterior neural tube (b'), and muscles (c'). These fluorescent signals are maintained in the hatchlings (d). In Tg[sox5-hs:GFP] embryos, GFP is expressed in a range of the central nervous system (CNS) from the forebrain to hindbrain (e'), and in neural tubes and the premigratory neural crest in a dotted manner (f'). At 9 dpf, additional fluorescent signals are observed in the pectoral fins, olfactory bulbs, and presumable xanthophore progenitors on the dorsal body surface (g; see also Additional file 3: Fig. S1C–D) |
|
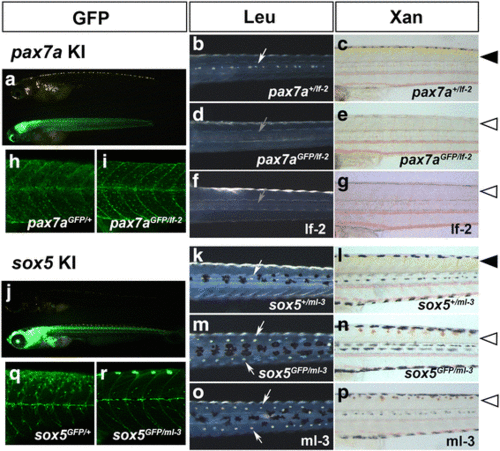
Disruption of gene function by knocking-in pax7a and sox5. (a-e, h-i) Progenies (9 dpf) of a pax7a-transgenic founder crossed to an existing pax7a mutant, leucophore free 2 (lf-2, f-g). (j-n, q-r) Progenies (9 dpf) of a sox5-transgenic founder crossed to an existing sox mutant, many leucophores 3 (ml-3, o-p). (a, h, i, j, q, r) GFP. (b, d, f, k, m, o) Leucophores viewed in reflected light. (c, e, g, l, n, p) Xanthophores viewed in transmitted light. (b, d, f, k, m, o) Dorsal views of the trunk. (c, e, g, h, i, l, n, p, q, r) Lateral views of the trunk. (h–i, q–r) Confocal microscopy of the body surface of the nine dpf progenies. (a, j) Lateral views of pax7a-transgenic fish (a) and sox5-transgenic fish (j). A non-GFP (upper) and a GFP-positive (lower) larva are shown. Whereas the non-GFP larva (pax7a +/lf-2 ) has leucophores in the dorsal midline (b, white arrow) and shows yellow pigmentation in the skin (c, arrowhead), presumably having xanthophores developing normally, the GFP-positive knock-in larva (pax7a GFP/lf-2 ) fails to have leucophores (d, grey arrow) or show yellow pigmentation (e, open arrowhead). An lf-2 larva shows the pax7a loss-of-function phenotypes (f, g). Whether having a pax7a knock-in GFP allele with a wild-type allele (h, heterozygote) or with an lf-2 allele (i, homozygote), the larva has GFP/pax7a-positive progenitors on the surface of the trunk. As shown in B and C, the non-GFP offspring obtained from the sox5-transgenic founder shows normal pigmentation (k, white arrow; l, arrowhead). The GFP-positive larva has excess leucophores bilaterally along the dorsal midline (m, arrows) and lacks yellow pigmentation (n, open arrowhead), as does the ml-3 mutant (o, p). While sox5 heterozygous larva (q, sox5 GFP/+ ) has GFP-positive presumable xanthophore progenitors on the surface of the trunk, sox5 homozygote (r, sox5 GFP/ml-3 ) lacks GFP-positive cells in the corresponding area |
|
Disruption of gene function by knocking-in pnp4a. (a, b) The expression pattern of GFP in founder fish. GFP expression was observed in the eye and abdomen. These fish were considered to have “good expression” of GFP, and were raised to adulthood. GFP expression was observed in the eye and abdomen. (c–f) The progenies (4 dpf) of a pnp4a-transgenic founder crossed to an existing gu mutant. All GFP-positive F1 fish have less-pigmented eyes and all GFP-negative F1 fish have wild-type pigmentation. Asterisks indicate GFP-positive and less-pigmented F1 fish |