- Title
-
Human TAUP301L overexpression results in TAU hyperphosphorylation without neurofibrillary tangles in adult zebrafish brain.
- Authors
- Cosacak, M.I., Bhattarai, P., Bocova, L., Dzewas, T., Mashkaryan, V., Papadimitriou, C., Brandt, K., Hollak, H., Antos, C.L., Kizil, C.
- Source
- Full text @ Sci. Rep.
|
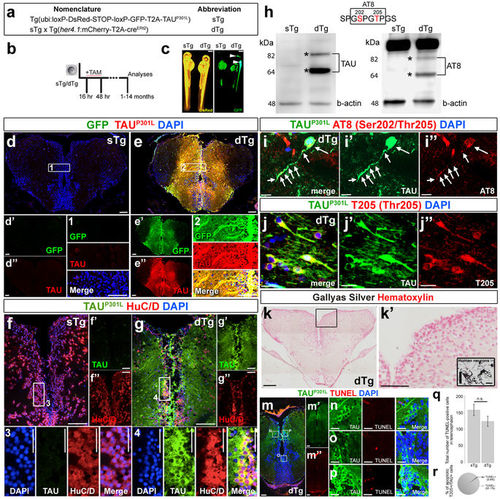
(a) Naming of transgenic constructs: sTg – effector transgenic, dTg – double transgenic. (b) Scheme for timing of recombination and analyses. (c) sTg and dTg animals (5 days post fertilization) treated with tamoxifen. DsRed expression indicates the presence of the effector cassette. Note the recombination in the central nervous system by GFP expression. (d) Immunohistochemistry (IHC) for GFP and TAUP301L on coronal sections of telencephalon of a 6 month-old sTg animal. Single channel images of the whole section for GFP (d’) and TAU (d”). (1) is the enlarged view of the inset in d. (e) IHC for GFP and TAUP301L on coronal sections of telencephalon of a 6-month old dTg animal. Single channel images of the whole section for GFP (e’) and TAU (e”). (2) is the enlarged view of the inset in f. (f) IHC for HuC/D and TAUP301L on coronal sections of telencephalon of an sTg animal. Single channel images of the whole section for TAU (f’) and HuC/D (f”). (3) is the enlarged view of the inset in f. (g) IHC for HuC/D and TAUP301L on coronal sections of telencephalon of a dTg animal. Single channel images of the whole section for TAU (g’) and HuC/D (g”). (4) is the enlarged view of the inset in g. (h) Western blot analyses for expression of TAUP301L (left) and hyperphosphorylated TAUP301L (right) in telencephalon. Beta actin is used as a loading control. (i) IHC for GFP and AT8 in a dTg animal. Individual channels are shown for GFP (i’) and AT8 (i”). Arrows represent the cytoplasmis signal. (j) IHC for TAUP301L and T205 in a dTg animal. Individual channels are shown for TAUP301L (j’) and T205 (j”). (k) Gallyas silver (black) and Hematoxylin (pink) staining in telencephalon of a dTg animal. (k’) Enlarged region in k’. Note the absence of Gallyas silver-positive cells. (l) Positive control for Gallyas silver staining in human neurons treated with Amyloid, showing neurofibrillary tangles. (m) Immunohistochemistry for TAUP301L (green) combined with TUNEL detection of apoptotic cells (red) in recombined 6 months old dTg animals. Individual fluorescent channels are shown in m’ and m”. (n–p) Higher magnification images from the insets in m. (q) Quantification of TUNEL-positive cells in the telencephalon in sTg and dTg animals. (r) Quantification of apoptotic cells containing hyperphosphorylated TAUP301L (T205-positive). Values represent mean ± s.e.m. *p < 0.05, **p < 0.01, ***p < 0.005. Scale bars equal 10 μm (i–j’) and 50 μm elsewhere. n = 6 fish and > 30 histological sections for every staining. Larvae are 5 days old, and adult animals are 6 months old. |
|
(a) Immunohistochemistry (IHC) for L-Plastin (red) and TAUP301L (green) on coronal sections of telencephalon of a 6 month-old sTg animal. Single channel images of the whole section for TAUP301L (a’) and L-Plastin (a”). (b) The enlarged view of the inset in a. (c) IHC for L-Plastin (red) and TAUP301L (green) on coronal sections of telencephalon of a 6-month old dTg animal. Single channel images of the whole section for TAUP301L (c’) and L-Plastin (c”). (d) The enlarged view of the inset in c. (e) Quantification of round and ramified L-Plastin-positive cells in the telencephalon in sTg and dTg animals. (f) Immunohistochemistry (IHC) for S100β (red), PCNA (green) and TAUP301L (white) on coronal sections of telencephalon of a 6-month old sTg animal. Single channel images of the whole section for S100β (F’), PCNA (f”), and TAUP301L (f”’). (g) IHC for S100β (red), PCNA (green) and TAUP301L (white) on coronal sections of telencephalon of a 6-month old dTg animal. Single channel images of the whole section for S100β (g’), PCNA (g”), and TAUP301L (g”’). (h) The enlarged view of the inset in f. (i) The enlarged view of the inset in g. (j) Quantification of the total number of proliferating glial cells in the telencephalon of sTg and dTg animals. Values represent mean ± s.e.m. *p < 0.05, **p < 0.01, ***p < 0.005. Scale bars equal 50 μm (a–g”’) and 20 μm (h,i). n = 7 fish and > 30 histological sections for every staining. All animals are 6 months old. |
|
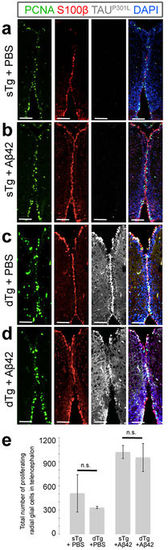
Immunohistochemistry for PCNA (green), S100β (red), and TAUP301L (white) on coronal sections of telencephalon of a 6 month-old sTg animal injected with PBS (a), sTg animal injected with Aβ42 (b), dTg animal injected with PBS (c), and dTg animal injected with Aβ42 (d). (e) Quantification of the total number of proliferating radial glial cells in the telencephalon of sTg and dTg animals injected with PBS or Aβ42. Values represent mean ± s.e.m. *p < 0.05, **p < 0.01, ***p < 0.005. Scale bars equal 100 μm. n = 5 fish and > 20 histological sections for every staining. All animals are 6 months old. |
|
(a) Immunohistochemistry (IHC) for TAUP301L (red), GFP (green) and L-Plastin (white) on coronal sections of telencephalon of a 6 month-old sTg animal injected with PBS (a), sTg animal injected with Aβ42 (b), dTg animal injected with PBS (c), and dTg animal injected with Aβ42 (d). Insets below the panels show individual fluorescent channels for TAUP301L (red), GFP (green) and L-Plastin (white). Columns 1–4 are single and merged images of the regions indicated in (a–d), respectively. (e) Quantification of the total number of ramified and round L-Plastin-positive cells in the telencephalon of sTg and dTg animals injected with PBS or Aβ42. (f–g) Immunohistochemistry for Interleukin-4 (red) on coronal sections of telencephalon of a 6-month old dTg animal injected with PBS (f) or with Aβ42 (g). (h) Relative change in the expression levels of il4 after Aβ42 injection compared to control injection. Medial ventricular regions are shown. DAPI (cyan) marks the nuclei. Values represent mean ± s.e.m. *p < 0.05, **p < 0.01, ***p < 0.005. Scale bars equal 50 μm. n = 6 fish and > 30 histological sections for every staining. All animals are 6 months old. |
|
(a) Immunohistochemistry (IHC) for TAUP301L (red), GFP (green) and L-Plastin (white) on coronal sections of telencephalon of a 6 month-old sTg animal injected with PBS (a), sTg animal injected with Aβ42 (b), dTg animal injected with PBS (c), and dTg animal injected with Aβ42 (d). Insets below the panels show individual fluorescent channels for TAUP301L (red), GFP (green) and L-Plastin (white). Columns 1–4 are single and merged images of the regions indicated in (a–d), respectively. (e) Quantification of the total number of ramified and round L-Plastin-positive cells in the telencephalon of sTg and dTg animals injected with PBS or Aβ42. (f–g) Immunohistochemistry for Interleukin-4 (red) on coronal sections of telencephalon of a 6-month old dTg animal injected with PBS (f) or with Aβ42 (g). (h) Relative change in the expression levels of il4 after Aβ42 injection compared to control injection. Medial ventricular regions are shown. DAPI (cyan) marks the nuclei. Values represent mean ± s.e.m. *p < 0.05, **p < 0.01, ***p < 0.005. Scale bars equal 50 μm. n = 6 fish and > 30 histological sections for every staining. All animals are 6 months old. |
|
(a) Immunohistochemical staining for TAUP301L on a sagittal section of a 3 month-old Tg(nbt:mCherry-t2a-creERT2) x Tg (ubi:loxPDsRed- loxP-GFP-t2a-TAUP301L) animal recombined as in Fig. 1. Inset shows the unrecombined animal. (b) Immunohistochemical staining for PCNA and S100β on a serial section of the same animal. (c) Immunohistochemical staining for L-Plastin on a serial section of the same animal. (d) Comparative quantification of the number of proliferating glial cells. (e) Comparative quantification of the relative numbers of L-Plastin-postive cells. Values represent mean ± s.e.m. n.s.: not significant. Scale bars equal 100 μm. |
|
(a) Immunohistochemistry (IHC) for TAUP301L (red) and GFP (green) on coronal sections of telencephalon of a 1 month-old nontransgenic animal. (a’, a’’) Individual fluorescent channels for TAUP301L (a’) and GFP (a’’). (1) The enlarged view of the inset in a. (b) IHC for TAUP301L and GFP on coronal sections of telencephalon of a 1-month old dTg animal. (b’, b’’) Individual fluorescent channels for TAUP301L (b’) and GFP (b’’). (2) The enlarged view of the inset in b. (c) IHC for TAUP301L and GFP on coronal sections of telencephalon of a 3-month old dTg animal. (c’, c’’) Individual fluorescent channels for TAUP301L (c’) and GFP (c’’). (3) The enlarged view of the inset in c. (d) IHC for TAUP301L and GFP on coronal sections of telencephalon of a 6-month old dTg animal. (d’, d’’) Individual fluorescent channels for TAUP301L (d’) and GFP (d’’). (4) The enlarged view of the inset in d. Scale bars equal 50 μm. n = 4 fish and >25 histological sections for every staining. |
|
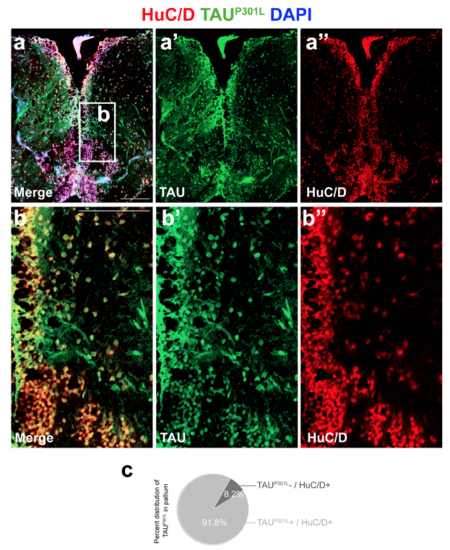
Immunohistochemistry for HuC/D (red) and TAUP301L (green) on the telencephalon of a 6 month-old dTg animal. (a’, a’’) Individual channels for green and red. (b-b’’) High-magnification image of the frame in a. (c) Quantification of the percentage of HuC/D-positive neurons expressing TAU in the pallium. Scale bars equal 100 μm. n = 4 fish and >20 histological sections for every staining and quantification. |
|
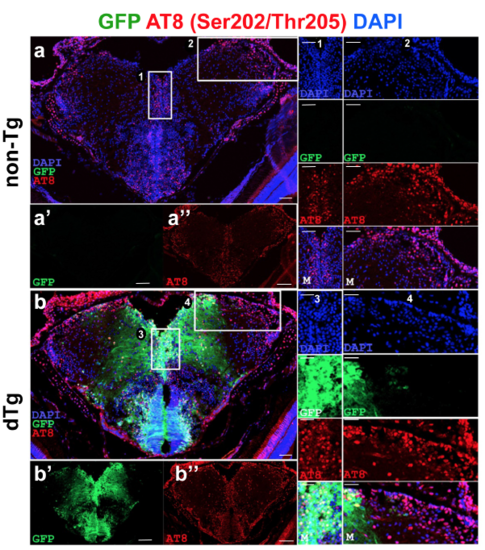
(a) Immunohistochemistry (IHC) for AT8 (red) and GFP (green) on coronal sections of telencephalon of a 6 month-old non-transgenic animal. (a’, a’’) Individual fluorescent channels for GFP (a’) and AT8 (a’’). (1 and 2) The enlarged view of the inset in A with individual channels for DAPI, GFP and AT8, and merged image. (b) IHC for AT8 and GFP on coronal sections of telencephalon of a 6-month old dTg animal. (b’, b’’) Individual fluorescent channels for GFP (b’) and AT8 (b’’). (3 and 4) The enlarged view of the inset in b with individual channels for DAPI, GFP and AT8, and merged image. Scale bars equal 25 μm. n = 5 fish for every staining. |
|
Immunohistochemical staining for GFP (recombined cells, white), T205 (hyperphosphorylated TAU, green), and TAUP301L (red) in 6 month-old dTg animal. (a) merge image. Individual channels for (b) GFP, (c) T205, and (d) TAUP301L. (e) Quantification of the percentage of TAUP301Lpositive cells that are T205 positive or negative. Scale bars equal 50 μm. n = 3 fish and >20 histological sections for every staining and quantification. |
|
(a) Immunohistochemistry (IHC) for AT180 (red) and GFP (green) on coronal sections of telencephalon of a 6-month old nontransgenic animal. (a’, a’’) Individual fluorescent channels for GFP (a’) and AT180 (a’’). Insets show the enlarged view of the frame in A with individual channels for DAPI, GFP and AT180, and merged image. (b) IHC for AT180 and GFP on coronal sections of telencephalon of a 6-month old dTg animal. (b’, b’’) Individual fluorescent channels for GFP (b’) and AT180 (b’’). Insets show the enlarged view of the frame in b with individual channels for DAPI, GFP and AT180, and merged image. (c) Western blot for AT180 from brains of non-Tg, sTg and dTg animals. Scale bars equal 25 μm. n = 5 fish for every staining. |
|
(a) Immunohistochemistry (IHC) for AT270 (red) and GFP (green) on coronal sections of telencephalon of a 6-month old nontransgenic animal. (a’, a’’) Individual fluorescent channels for GFP (a’) and AT270 (a’’). Insets show the enlarged view of the frame in a with individual channels for DAPI, GFP and AT270, and merged image. (b) IHC for AT270 and GFP on coronal sections of telencephalon of a 6-month old dTg animal. (b’, b’’) Individual fluorescent channels for GFP (b’) and AT270 (b’’). Insets show the enlarged view of the frame in b with individual channels for DAPI, GFP and AT270, and merged image. (c) Western blot for AT270 from brains of non-Tg, sTg and dTg animals. Scale bars equal 50 μm. n = 6 fish for every staining. |
|
(a-d) Immunohistochemistry (IHC) for TAUP301L (green) and staining for apoptotic cells with TUNEL (green) telencephalon of a 6- month old non-transgenic animal injected with Amyloid-beta42. (a-c) Individual fluorescent channels for DAPI (a), TAUP301L (b), and TUNEL (c). (d) Merged image. (e-h) IHC for TAUP301L (green) and staining for apoptotic cells with TUNEL (green) telencephalon of a 6-month old dTg animal. (a-c) Individual fluorescent channels for DAPI (e), TAUP301L (f), and TUNEL (g). (h) Merged image. (i) Quantification of relative change in the expression levels of casp3 mRNA in dTg compared to sTg adult zebrafish brains. Scale bars equal 25 μm. n = 5 fish for every staining. |
|
(a) Immunohistochemistry (IHC) for TAUP301L (red) and GFP (green) on coronal sections of telencephalon of a 14-month old sTg animal. (b,c) Individual fluorescent channels for TAUP301L (b) and GFP (c). (d) IHC for TAUP301L and GFP on coronal sections of telencephalon of a 14 month-old dTg animal. (e,f) Individual fluorescent channels for TAUP301L (e) and GFP (f). (g) IHC for L-Plastin and TAUP301L on coronal sections of telencephalon of a 14 month-old sTg animal. (h,i) Individual fluorescent channels for TAUP301L (h) and L-Plastin (i). (j) IHC for L-Plastin and TAUP301L on coronal sections of telencephalon of a 14-month old dTg animal. (k,l) Individual fluorescent channels for TAUP301L (k) and L-Plastin (l). (m) Quantification of round and ramified L-Plastin-positive cells in the telencephalon of 14-month-old sTg and dTg animals. Values represent mean ± s.e.m. *: p<0.05, **: p<0.01, ***: p<0.005. Scale bars equal 50 μm. n = 5 fish and >25 histological sections for every staining. |
|
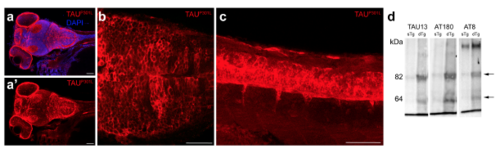
(a) Immunohistochemical staining for TAUP301L in 9 day-old dTg larvae. Anterior view of the brain is shown. (b) Single fluorescence channel for TAUP301L. (c) High-magnification image of the frame in b. (d) TAUP301L expression in the spinal cord. (e) Western blot analyses of TAUP301L (TAU13), and hyperphosphorylated form of TAU with AT180 and AT8. Scale bars equal 50 μm. |
|
Immunohistochemical staining for TAUP301L (red), GFP (green), and T22 (TAU oligomers, gray) in sTg (a-d), dTg (e-h), sTg injected with Aβ42 (i-l), and dTg injected with Aβ42 (m-p). Scale bars equal 50 μm. All animals are 6 month-old. |