- Title
-
Zebrafish heart regeneration: 15 years of discoveries
- Authors
- González-Rosa, J.M., Burns, C.E., Burns, C.G.
- Source
- Full text @ Regeneration (Oxf)
|
Causes and consequences of myocardial infarction in mammals. (A) Schematic representation of a human heart in which one of the coronary arteries is occluded by an atheromatous plaque (magnified area). When blood flow is interrupted, a region of the myocardium becomes ischemic (brown shade). Ischemic myocardium eventually dies and is replaced by fibrotic tissue. (B) Anatomical and histological differences between a healthy and an infarcted heart. In contrast to a healthy heart, the infarcted ventricle shows a thinning of the affected wall, in which the cardiac muscle has been replaced by fibrotic tissue. LV, left ventricle; RV, right ventricle |
|
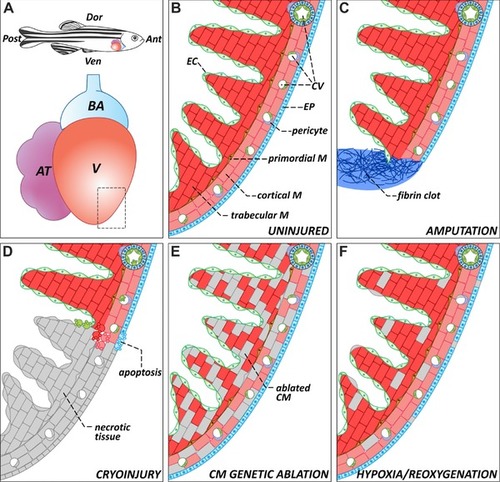
The zebrafish heart: anatomy, histology, and injury paradigms. (A) Schematic representation of the anatomical position of the heart in the adult zebrafish. The teleost heart is composed of a single atrium and a single ventricle. Blood exits the heart through the bulbus arteriosus, an elastic, non‐contractile chamber composed of smooth muscle. (B) Histological organization of the adult zebrafish ventricle. Cardiac muscle is covered externally by the epicardium and internally by the endocardium. The myocardium is divided into three distinctive populations: trabecular, primordial, and cortical. The cortical myocardium is highly irrigated by coronary vessels. Endothelial cells from the coronary vasculature are frequently surrounded by pericytes. For simplicity, the presence of fibroblasts in the uninjured heart has been omitted. (C) Apex amputation removes ∼20% of the ventricle and leads to the formation of a fibrin clot. (D) Cryoinjury induces local tissue necrosis (∼20% of the ventricle) and triggers apoptosis. (E) Cardiomyocyte genetic ablation causes diffuse loss of ∼60% of cardiomyocytes in the heart, while preserving the remaining cell types. (F) Hypoxia/reoxygenation induces low levels of diffuse cell death in all cell types of the heart. Ant, anterior; AT, atrium; BA, bulbus arteriosus; CM, cardiomyocyte; CV, coronary vasculature; Dor, dorsal; EC, endocardium; EP, epicardium; M, myocardium; Post, posterior; V, ventricle; Ven, ventral |
|
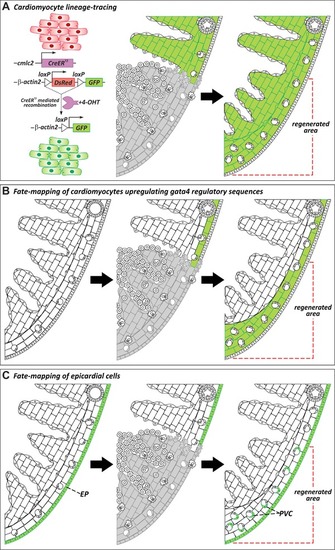
Cellular origins of regenerated tissue. (A) Genetic lineage‐tracing experiments to determine the origin of regenerated myocardium during zebrafish heart regeneration. Virtually all cardiomyocytes in uninjured hearts are labeled by GFP expression after treatment with 4‐hydroxytamoxifen (4‐OHT). In regenerated hearts, the new myocardium is GFP+, revealing that new muscle derives from the proliferation of preexisting cardiomyocytes (Jopling et al., |
|
Dynamics of zebrafish heart regeneration. Representations of a region of the zebrafish heart in the absence of injury (A) or at different stages after cryoinjury (B−F). (B) Ventricular cryoinjury induces local tissue necrosis (gray) and apoptosis in all cell types around the injured region. Tissue death triggers the recruitment of inflammatory cells and endocardial activation. (C) During the first days after injury, epicardial and endocardial cells proliferate actively and cover the injured area, establishing a “regenerative scaffold.” Epicardial cells also undergo epithelial to mesenchymal transitions and invade the underlying myocardium. Myofibroblasts appear in the injury zone and there is an accumulation of extracellular matrix. (D−E) Cardiomyocytes located in the wound edge proliferate and repopulate the injured area. As the myocardium regenerates, the fibrotic tissue progressively disappears. (F) In advanced stages of regeneration, the zebrafish myocardium appears completely restored. Compared to uninjured controls or to the contralateral wall, the regenerated wall shows a significant expansion of the cortical myocardium. dpi, days post‐injury; ECM, extracellular matrix; EP, epicardium; PVC, perivascular cell |