- Title
-
A GEF activity-independent function for nuclear Net1 in Nodal/Smad2 signal transduction and mesendoderm formation
- Authors
- Wei, S., Ning, G., Li, L., Yan, Y., Yang, S., Cao, Y., Wang, Q.
- Source
- Full text @ J. Cell Sci.
|
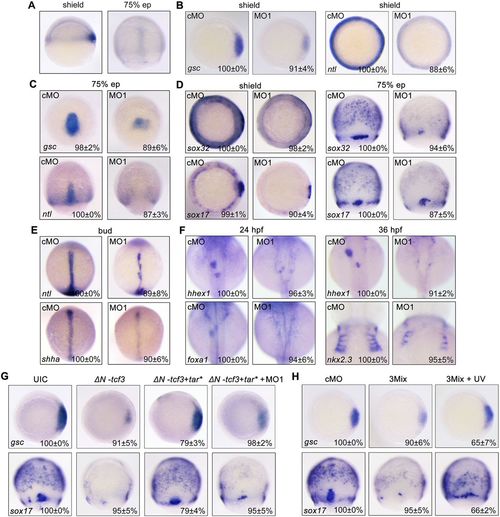
net1 is crucial for mesendoderm formation in zebrafish embryos. (A) Visualization of net1 expression in zebrafish embryos at shield and 75% epiboly (ep) stages using whole-mount in situ hybridization. Shield stage, lateral views with dorsal to the right; 75% epiboly stage, dorsal views with animal pole to the top. (B–F) The expression of mesendoderm marker genes and mesendoderm derivative marker genes in cMO- and net1 MO1-injected embryos at the indicated stages. (B–D) Shield stage, animal views with dorsal to the right; 75% epiboly stage, dorsal views with animal pole to the top. (E,F) Bud stage, and 24 and 36 hpf. All images are shown in dorsal views with anterior to the top. (G) Wild-type embryos were injected with 50 pg ΔN-tcf3 mRNA alone or co-injected with 3 pg tar* mRNA and 4 ng net1 MO1, and then collected at the shield and 75% epiboly stages for whole-mount in situ hybridization with a gsc or sox17 probe. (H) Overexpression of net1 rescues mesendoderm induction defects in net1 morphants. Wild-type embryos were injected with cMO or net1 3Mix (4 ng net1 MO1, 200 pg Flag-net1 mRNA and 1 ng AS-Flag-photo-MO), and then a subset of net1 3Mix-injected embryos were exposed to UV light at the sphere stage. The expression of gsc and sox17 was examined by in situ hybridization at shield and 75% epiboly stages, respectively. In B–H, the percentages (mean±s.d.) of the affected embryos are shown as calculated from three independent biological repeats with ∼15–30 embryos in each group. UIC, uninjected control. |
|
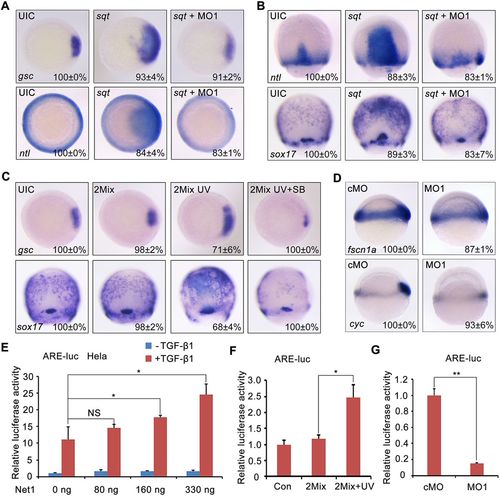
Net1 upregulates Nodal signal transduction. (A,B) Knockdown of net1 decreases Nodal ligand-induced mesendoderm expansion. Wild-type embryos were injected with 1 pg sqt mRNA alone or concurrently with 4 ng net1 MO1 at the one-cell stage, harvested at the shield and 75% epiboly stages, and analyzed for the expression of indicated marker genes by using whole-mount in situ hybridization. (C) Zebrafish embryos were injected with net1 2Mix (300 pg Flag-net1 mRNA and 1 ng AS-Flag-photo-MO) at the one-cell stage. A subset of these embryos was treated with 25 μM SB431542 (SB) from the 64-cell stage, of which a portion was exposed to UV light at the sphere stage. The expression of gsc and sox17 was examined by in situ hybridization at the shield and 75% epiboly stages, respectively. (D) The expression of Nodal target genes cyc and fscn1a in cMO- and net1 MO1-injected embryos at shield stage. Lateral view with animal pole to the top. In A–D, the percentages (mean±s.d.) of the affected embryos are shown as calculated from three independent biological repeats with ∼15–25 embryos in each group. UIC, uninjected control. (E,F) Overexpression of Net1 enhanced ARE-luciferase expression. (E) HeLa cells were transfected with the ARE-luciferase reporter concurrently with increasing amounts of Net1. At 36 h post transfection, the cells were treated with TGF-β1 or left untreated overnight, and then harvested for luciferase assays. *P<0.05; NS, not significant (Student's t-test). (F) At the one-cell stage, zebrafish embryos were injected with the ARE-luciferase reporter alone or together with net1 2Mix. At the sphere stage, half of the net1 2Mix-injected embryos were exposed to UV light, and then all embryos were harvested at the shield stage for luciferase assays. *P<0.05 (Student's t-test). (G) Knockdown of net1 inhibits ARE-luciferase expression. At the one-cell stage, zebrafish embryos were co-injected with the ARE-luciferase reporter and cMO or net1 MO1, and then harvested at the shield stage for luciferase assays. **P<0.01 (Student's t-test). Results in E–G are mean±s.d. (n=3). |
|
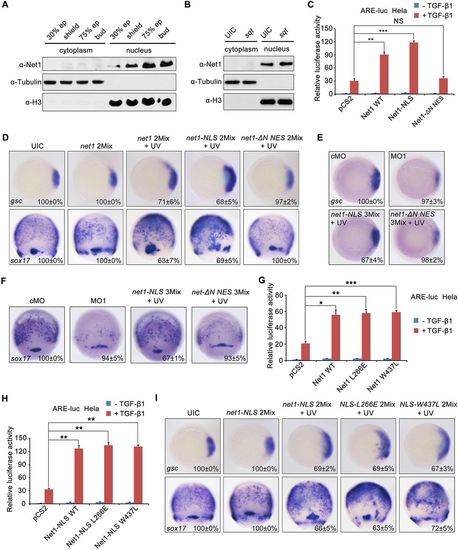
Nuclear Net1 enhances Nodal signaling and mesendoderm formation in a GEF-independent manner. (A,B) Endogenous Net1 primarily localizes in the zebrafish embryonic cell nucleus. Nuclear and cytosolic fractions were extracted from wild-type embryos at indicated stages (A) or from sqt mRNA-injected embryos at the shield stage (B). Then these resulting samples were assessed by western blotting with the indicated antibodies. (C) Net1 promotes Nodal signaling in the nucleus. HeLa cells were transfected with the ARE-luciferase reporter together with Net1, Net1-NLS or Net1-ΔN-NES. After 36 h of transfection, the cells were treated with or without TGF-β1 overnight, then harvested for luciferase assays. **P<0.01; ***P<0.001; NS, not significant (Student's t-test). (D) Nuclear Net1 facilitates mesendoderm induction. At the sphere stage, zebrafish embryos injected with net1 2Mix or net1-NLS 2Mix or net1-ΔN NES 2Mix were exposed to UV light, and then harvested for whole-mount in situ hybridization to detect the expression of gsc and sox17 at the shield and 75% epiboly stages. (E,F) Overexpression of the nuclear, but not cytosolic, Net1 rescues the mesendoderm defects in net1 morphants. Embryos were injected with cMO, net1 MO1, net1-NLS 3Mix or net1-ΔN NES 3Mix. Embryos injected with net1-NLS 3Mix or net1-ΔN NES 3Mix were exposed to UV light at the sphere stage. The expression of gsc (E) and sox17 (F) was examined by in situ hybridization at the shield and 75% epiboly stages, respectively. (G,H) Net1 promotes Nodal signaling independently of its GEF activity. HeLa cells were co-transfected with the ARE-luciferase reporter and wild-type Net1 and its GEF-deficient mutants (G) or nuclear Net1 and its GEF-deficient mutants (H). After 36 h of transfection, the cells were treated with or without TGF-β1 overnight, then harvested for luciferase assays. *P<0.05, **P<0.01, ***P<0.001 (Student's t-test). (I) The influence of nuclear Net1 and its GEF-deficient mutants in mesendoderm formation. Zebrafish embryos were injected with net1-NLS 2Mix, NLS-L266E 2Mix and NLS-W437L 2Mix, respectively. These embryos were exposed to UV at the sphere stage, then harvested for whole-mount in situ hybridization. In D,E,F and I, the percentages (mean±s.d.) of the affected embryos are shown as calculated from three independent biological repeats with ∼20–30 embryos in each group. Results in C,G and H are mean±s.d. (n=3). UIC, uninjected control. EXPRESSION / LABELING:
PHENOTYPE:
|
|
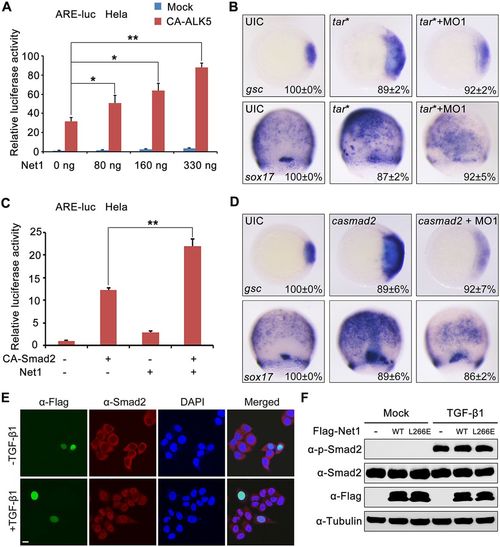
Net1 promotes Nodal signal downstream of or parallel to Smad2. (A) Overexpression of Net1 promotes CA-ALK5-induced ARE-luciferase expression. At 36 h after transfecting with ARE-luciferase reporter together with increasing amounts of Net1 expression plasmids, HeLa cells were re-transfected with or without CA-ALK5 for 24 h, and then harvested for luciferase assays. *P<0.05; **P<0.01 (Student's t-test). (B) One-cell-stage zebrafish embryos were injected with 3 pg tar* mRNA alone or together with 4 ng net1 MO1, and then harvested for whole-mount in situ hybridization. (C) HeLa cells were co-transfected with the ARE-luciferase reporter and indicated plasmids. At 36 h after transfection, the cells were harvested for luciferase assays. **P<0.01 (Student's t-test). (D) One-cell-stage zebrafish embryos were injected with 25 pg ca-smad2 mRNA alone or together with 4 ng net1 MO1, and then harvested for whole-mount in situ hybridization. (E) Overexpression of Net1 does not influence the subcellular localization of Smad2. HeLa cells transfected with Flag–Net1 were treated with or without TGF-β1 for 1 h, and then immunostained with anti-Flag (green) and anti-Smad2 (red) antibodies. Nuclei were counterstained with DAPI and are shown in blue. Scale bar: 10 μm. (F) Overexpression of Net1 does not affect the phosphorylation of Smad2. HeLa cells were transfected with Flag-tagged wild-type (WT) Net1 or the Net1-L266E mutant. After 36 h of transfection, these cells were treated with TGF-β1 or left untreated for 4 h, and then harvested for western blotting using the indicated antibodies. Results in A and C are mean±s.d. (n=3). In B and D, the percentages (mean±s.d.) of the affected embryos are shown as calculated from three independent biological repeats with ∼20–25 embryos in each group. UIC, uninjected control. |