- Title
-
Light-Dependent Regulation of Sleep and Wake States by Prokineticin 2 in Zebrafish
- Authors
- Chen, S., Reichert, S., Singh, C., Oikonomou, G., Rihel, J., Prober, D.A.
- Source
- Full text @ Neuron
|
prok2 Expression in the Larval Zebrafish Brain (A and B) prok2 mRNA (black box and arrow) is expressed in a bilateral cluster of approximately ten neurons in the ventral hypothalamus at 5 dpf. Dashed box in (A) indicates the region shown in (D). In (B), only the purple stain indicated by the arrow near the ventral brain surface is specific staining. (C and C′) Schematic drawings summarize gene expression patterns from (E)–(H), as well as brain regions not shown in those panels. Dashed black circles indicate anatomical region potentially analogous to the mammalian SCN. (D) prok2-expressing neurons co-express vglut1. DAPI nuclear stain is shown in blue. A representative cell, indicated by a white arrow, is enlarged in the inset. A 0.46-μm confocal section is shown. (E–H) In the ventral hypothalamus, prok2 is expressed caudal and dorsal to populations that express avp (E) and caudal to cells that express vip (F). grp (G) and tgfα (H) are not expressed in the ventral hypothalamus. 34- (E–G) and 60- (H) μm-thick confocal projections are shown. (E′′′), (F′′′), (G′′′), and (H′′′) show orthogonal projections of each confocal stack. All samples are at 5 dpf. Ventral views with rostral at top (A, C–H, D′–H′, D″–H″) and lateral views with rostral at top and dorsal at right (B, C′, E′′′–H′′′) are shown. Scale bars in (A) and (B), 100 μm, and in (D)–(H), 10 μm. See also Figure S1. EXPRESSION / LABELING:
|

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. |

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. |

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. |

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. |

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. |
|
Expression Patterns of prokr1 and prokr2 in Larval Zebrafish (A and B) prokr1 is expressed in a row of cells in the spinal cord at 24 hpf (A, enlarged in inset) and in a bilateral cluster of cells in the ventral hypothalamus at 5 dpf (arrow in A′). Most of the embryo in (A) is overstained in order to see the weak prokr1-expressing cells in the spinal cord. prokr2 is expressed in discrete clusters of cells in the hindbrain (B), midbrain (B′ and B″), and forebrain (B′ and B″) at 5 dpf. Arrows indicate domains of prokr2-expressing cells that are shown at higher magnification in the indicated panels. Side (A), ventral (A′, B′, and B″), and dorsal (B) views are shown, with rostral at top. (B′) and (B″) show dorsal and ventral optical sections, respectively. (C–H) prokr2-expressing neurons express vesicular glutamate transporter 2b (vglut2b) in the thalamic region (C) and glutamate decarboxylase (gad65 and gad67 probes combined) in the forebrain (E). prokr2-expressing neurons in the locus coeruleus (D) and medulla oblongata (F) express vmat:EGFP, which labels noradrenergic neurons. prokr2 is expressed in a subset of hypothalamic neurons that express hdc (G) and galn (H). Images show 0.46- (C and E), 10- (D and F), 2.3- (G), and 2.9- (H) μm-thick confocal projections. Ventral views are shown in (C)–(H), (C′)–(H′), and (C″)–(H″). (D′′′), (F′′′), (G′′′), and (H′′′) show orthogonal views of (D″), (F″), (G″), and (H″) at positions indicated by red lines. Arrows in (D) and (F)–(H) indicate representative neurons. In (C)–(H), samples were fixed at 5 dpf and are oriented with rostral at top. Scale bars in (A) and (B), 100 μm, and in (C)–(H), 10 μm. See also Figures S6 and S7. EXPRESSION / LABELING:
|
|
Light-Dependent Sleep Induction by Prok2 Is Correlated with Increased galn Expression and Requires galn (A–D) 29-μm-thick projections from representative brains showing galn expression in the anterior hypothalamus. Larvae were raised in LD until 5 dpf and then were not heat shocked (A and A′), heat shocked from 3 to 4 p.m. and kept in light (B and B′), or heat shocked from 3 to 4 p.m. and kept in dark (C and C′) and then fixed at 8 p.m. (D and D′) WT larvae were treated with either DMSO vehicle or 20 μM melatonin at 4 p.m. and fixed at 8 p.m. Ventral views with rostral at top are shown. (E) galn ISH in a 5-dpf brain. Red box indicates the area shown in (A)–(D) and quantified in (F). (F) Mean ± SEM fluorescence intensity of galn ISH. ∗∗∗p < 0.001 by two-way ANOVA for pairwise comparisons of the mean of each control sample (blue) with its corresponding hsp:Prok2 or melatonin-treated sample (red), with Bonferroni correction for multiple comparisons. White numbers indicate number of animals. Scale bar in (A)–(D), 50 μm, and in (E), 100 μm. (G) Amino acid sequences of WT and mutant zebrafish Galn (isoforms a and b). Blue and red boxes indicate Galn peptide and Galn message-associated peptide, respectively. Gray shading indicates altered amino acids in the mutant. (H–K) Larval progeny from a Tg(hsp:Prok2)/+; galn+/− to galn+/− mating were heat shocked (gray bar in H and J) during the day. This resulted in a significant decrease in locomotor activity (H and I) and increase in sleep (J and K) in Tg(hsp:Prok2)/+; galn+/+ and Tg(hsp:Prok2)/+; galn+/− animals compared to their non-transgenic galn−/−, galn+/−, and galn+/+ siblings. This phenotype was significantly suppressed in Tg(hsp:Prok2)/+; galn−/− animals, particularly following 1 hr after heat shock, reaching non-transgenic control levels after 3 hr. Line and bar graphs show mean ± SEM from a representative experiment containing two plates of four experiments, with different heat-shock times that preclude combining datasets from different experiments. n.s., not significant, ∗p < 0.001, ∗∗p < 1 × 10−10 by one-way ANOVA with post hoc Tukey’s test. See also Figure S8. |
|
prok2 and prokr2 expression levels do not oscillate in circadian manner, prok2 expression level is not affected by light, and changes in light do not activate prok2- expressing neurons in larval zebrafish. Related to Figure 1. (A, F) qRT-PCR analysis of prok2 and per3 (A), and prokr2 and per3 (F), mRNA is shown. Larvae were raised in 14:10 hr LD conditions and collected at the indicated times beginning at 6 dpf. Mean ± SEM for triplicate biological samples normalized to actin at each time point is shown. prok2 and prokr2 expression does not change significantly over 24 hr, while per3 expression oscillates with a 24 hr period. P values were calculated using one-way ANOVA. (B) 53 μm thick confocal projections showing prok2 FISH in larval zebrafish brains fixed at the indicated times starting at 6 dpf. Larvae were raised as described above. (C) Quantification of total prok2 fluorescence pixel intensity. prok2 expression does not oscillate (peak:trough ratio=1.24, p=0.40 by one-way ANOVA). Mean ± SEM for five brains at each time point is shown. a.u. = arbitrary units. (D) A 53 μm thick confocal projection showing prok2 FISH in brains of larvae raised in LL or DD and fixed at 6 dpf. (E) Mean ± SEM prok2 FISH fluorescence intensity for 5 brains in each condition, normalized to the LL condition. There is no significant difference in prok2 mRNA level between the two conditions (p=0.40 by two-tailed Student’s t test). (G, H) Larvae were raised until 6 dpf in either DD or LL, then exposed to light or dark, respectively. Samples were fixed at the indicated times after the change in lighting condition. Control larvae were maintained in the original lighting condition. qRT-PCR was performed to measure the level of prok2 (G) or prokr2 (H) mRNA. Mean ± SEM for triplicate biological samples normalized to actin at each time point is shown. Statistical significance was assessed by two-tailed Student’s t test. (I-L) prok2- expressing neurons do not express c-fos in response to changes in lighting conditions. Larvae raised in DD (I, J) or LL (K, L) until 5 dpf were transferred to light (I, J) or dark (K, L) conditions, and fixed 15 or 30 min later. Double FISH using prok2- and c-fos-specific probes showed that c-fos is not expressed in prok2-expressing neurons in either case. Scale bars: 10 μm. |
|
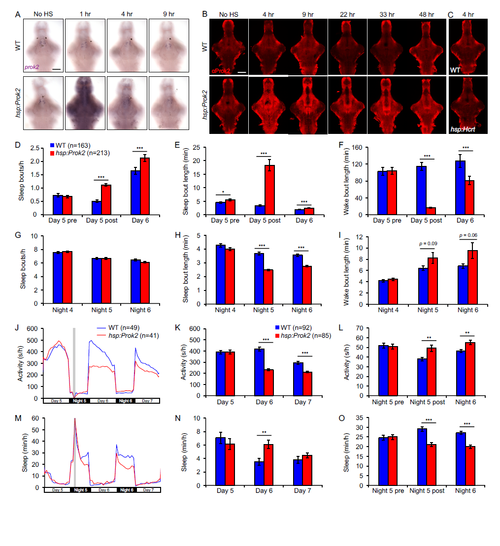
Dynamics of Prok2 overexpression, effects of induction of Prok2 overexpression during the day on sleep architecture, and induction of Prok2 overexpression at night produces a phenotype similar to that of daytime induction. Related to Figure 2. (A, B) Time course of prok2 mRNA and protein overexpression. Representative images of ISH using a prok2- specific probe (A) or IHC using a Prok2-specific antibody (B) in WT and Tg(hsp:Prok2) larvae. Larvae were heat shocked from 1-2 p.m. at 5 dpf and fixed at the indicated times after heat shock. Ectopic prok2 mRNA is detected for at least 4 hr after heat shock. Ectopic Prok2 protein can be detected for at least 48 hr after heat shock. (C) Representative images of IHC using a Prok2- specific antibody on 5 dpf WT and Tg(hsp:Hcrt) larvae fixed 4 hr after heat shock. Overexpressed Hcrt protein (Prober et al., 2006) is not detected by the Prok2-specific antibody. Brains were genotyped by PCR after imaging. No HS, no heat shock. Scale bars: 100 μm. (D-I) Following a daytime heat shock, increased daytime sleep due to Prok2 overexpression results from an increase in the number (D) and length (E) of sleep bouts, with a corresponding decrease in the length of wake bouts (F). Decreased sleep at night due to Prok2 overexpression results from a decrease in the length of sleep bouts (H). (J-O) Heat shock (gray bar in [J, M]) at night results in more activity at night (J, L) and less activity during the day (J, K) for Tg(hsp:Prok2) larvae compared to their WT siblings. Prok2 overexpression also results in less sleep at night (M, O) and more sleep during the day (M, N). These phenotypes were observed for up to 36 hr following heat shock. Note that (K, L, N, O) exclude the first two hr after heat shock to allow larvae to recover from the heat shock. Both genotypes slept more during this recovery period. Data from one representative experiment (J, M), two experiments combined (K, L, N, O) and five experiments combined (D-I) are shown. (D-I, K, L, N, O) show mean ± SEM. n = number of larvae. *p<0.05, **p<0.01, ***p<0.001 by two-tailed Student’s t test. |
|
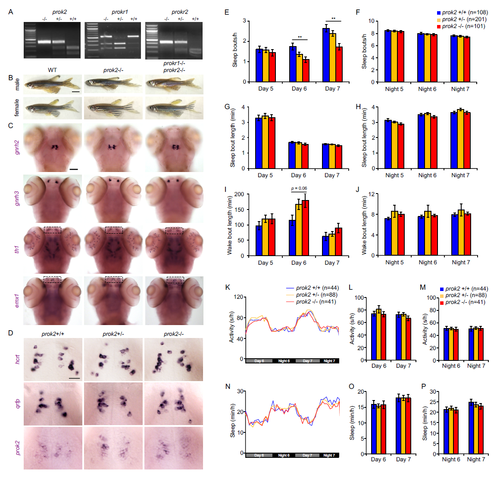
prok2 and prok receptor mutants lack development defects associated with Kallmann syndrome, sleep architecture of prok2 mutant larvae, and prok2 mutant larvae exhibit normal circadian regulation of locomotor activity and sleep. Related to Figures 5 and 6. (A) Representative genotyping images for prok2, prokr1 and prokr2 mutants. Bands for homozygous mutant, heterozygous mutant and homozygous WT are described in STAR Methods. (B) Images of representative WT, prok2-/-, and prokr1-/-; prokr2-/- 6-month old adult zebrafish. All fish are similar in size and morphology. (C) Representative images of ISH using probes specific for gnrh2, gnrh3, th1 and emx1 in 5 dpf WT, prok2-/-, and prokr1-/-; prokr2-/- larvae. Dashed boxes indicate olfactory region. (D) Representative images of ISH using probes specific for the hypothalamic neuropeptides hcrt, qrfp and prok2 in prok2+/+, prok2+/- and prok2-/- larvae. Larvae of all genotypes show similar expression levels and patterns of each gene. There is no apparent nonsense-mediated decay of prok2 mRNA in prok2 mutants. Animals were genotyped by PCR after imaging. Scale bars: (B) 5 mm, (C) 100 μm, (D) 10 μm. (E-J) Sleep architecture of prok2 mutant larvae. The increased activity and decreased sleep during days 6 and 7 of development in prok2-/- larvae is due to a decrease in the number of sleep bouts (E), with a trend towards longer waking bouts (I). (K-P) Larvae entrained in 14:10 hr LD conditions for 5 days and then monitored in DD maintain normal circadian rhythms of locomotor activity (K-M) and sleep (N-P), with no significant differences between prok2-/-, prok2+/- or prok2+/+ siblings. Data from 5 experiments (E-J) and 2 experiments (K-P) combined are shown. Bar graphs show mean ± SEM. n = number of larvae. **p<0.01 by one-way ANOVA, with post-hoc Dunnett’s test compared to WT. |

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. |
|
Effects of prok2 gain- and loss-of-function on hypothalamic galn expression, and prok2-expressing neuron fibers are present in the optic tectum. Related to Figure 8. (A, B) Prok2 overexpression increases hypothalamic galn expression in prokr2+/- larvae maintained in light for 4 hr after heat shock, but this effect is abolished in prokr2-/- larvae. (A) 28.8 μm thick confocal projections from representative brains showing hypothalamic galn FISH in Tg(hsp:Prok2) and non-transgenic prokr2+/- or prokr2-/- siblings. Animals were heat shocked at 6 dpf from 2-3 p.m. and then kept in light until fixation 4 hr later. (B) Mean ± SEM total fluorescence intensity of galn FISH. At least 7 brains were quantified for each condition. *p<0.05 by two-way ANOVA, with post-hock Tukey’s HSD test. (C, D) Prok2 overexpression induces galn expression in a light-specific manner. (C) 28.8 μm thick confocal projections from representative brains showing hypothalamic galn FISH. Tg(hsp:Prok2) and non-transgenic siblings were heat shocked at 6 dpf from 2-3 p.m. and then kept in light until 7 p.m. (4 hr on). Lights were then turned off for 1 hr (5 hr off), on for 1 hr (6 hr on) and off for 1 hr (7 hr off), and samples were fixed at the end of each hr. On and off refer to samples that were exposed to light or dark during the hr before fixation. (D) Mean ± SEM total fluorescence intensity of galn FISH. galn mRNA levels were only significantly higher in Prok2-overexpressing larvae that were exposed to light during the hr before fixation. At least 9 brains were analyzed for each condition. **p<0.01 by two-tailed Student’s t test. (E, F) prok2 mutant larvae have normal hypothalamic galn expression levels. (E) 28.8 μm thick confocal projections from representative brains showing hypothalamic galn expression in prok2+/+, prok2+/- and prok2-/- larvae that were raised in LD until 6 dpf and fixed at 10 a.m. (day) or 12 a.m. (night). These time points are 1 hr after lights on and off, respectively. (F) Mean ± SEM total fluorescence intensity of galn FISH. galn mRNA levels are not significantly different among the genotypes during the day or night (p>0.05 by two-way ANOVA). At least 7 brains were analyzed for each condition. (G-J) Fibers from prok2-expressing neurons are present in the optic tectum in larval zebrafish. (G) A 20 μm thick confocal projection showing the hypothalamus of a WT larva injected with a prok2:GFPAequorin transgene, fixed at 5 dpf and analyzed by double FISH using probes specific for prok2 (red) and gfp (green). (G'") shows an orthogonal view of this image at the position of the blue line in (G"), indicating co-localization of signals from the two probes. Arrows indicate examples of cells that express both genes. (H) Quantification showing that 98% of gfp-aequorin expressing cells co-express prok2, indicating that the transgene is specifically expressed in prok2-expressing neurons. Mean ± SEM for 7 animals is shown. (I) A 130 μm thick confocal projection showing the brain of a WT larva injected with the prok2:GFP-Aequorin transgene, fixed at 5 dpf and labeled with a GFP-specific antibody. The GFP-labeled neurons project extensively within the hypothalamus. (I’) shows an orthogonal view of this image, revealing dorsal projections to the optic tectum. (J) A 10 μm thick confocal section showing GFP-labeled fibers in the optic tectum. Yellow dashed lines outline the left and right optic tectum. Scale bars: (A, C, E) 50 μm, (G) 10 μm, (I) 100 μm. Anterior, posterior and dorsal axes are indicated. |