- Title
-
Reciprocal analyses in zebrafish and medaka reveal that harnessing the immune response promotes cardiac regeneration
- Authors
- Stainier, D.Y., Lai, S.B., Marín-Juez, R., Moura, P.L., Kuenne, C., Lai, J.K.H., Tsedeke, A.T., Guenther, S., Looso, M.
- Source
- Full text @ Elife
|
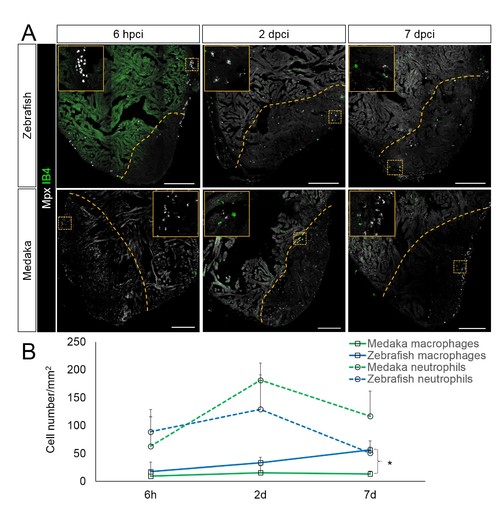
Immune cells dynamics in cryoinjured hearts. (A) Zebrafish and medaka heart sections at 6 hpci, 2 dpci and 7 dpci were stained with isolectin-B4 (IB4) for macrophages, and Mpx antibody for neutrophils. Positive cells, both in the injured area itself and within 100 μm of the injured area, were quantified (B; n = 3). Dotted lines delineate the injured area; scale bars, 200 μm. Macrophage numbers in medaka were always lower than those in zebrafish and this difference was especially pronounced at 7 pd. Neutrophil numbers appeared similar at 6 hpci, were higher in medaka at 2 dpci, and their clearance was delayed in medaka compared to zebrafish. |
|
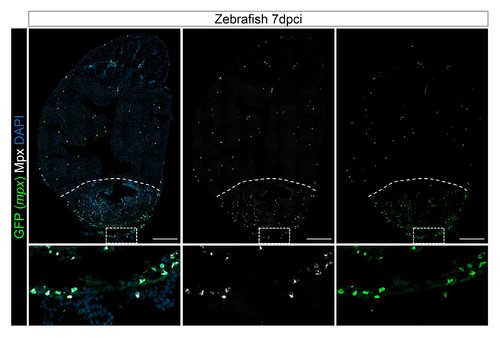
Mpx antibody specifically stains mpx:GFP-expressing cells. Injured hearts from adult mpx:GFP zebrafish were collected at 7 dpci, and cryosections stained with GFP and Mpx antibodies to examine neutrophils. Dotted lines delineate the injured area; scale bars, 200 μm. Percentage of Mpx+;GFP+ cells/GFP+ cells was quantified. 88.18 ± 0.57% of GFP+ cells were Mpx+, and we did not observe any Mpx-;GFP+ cells (Figure 3—figure supplement 1—source data 1). |
|
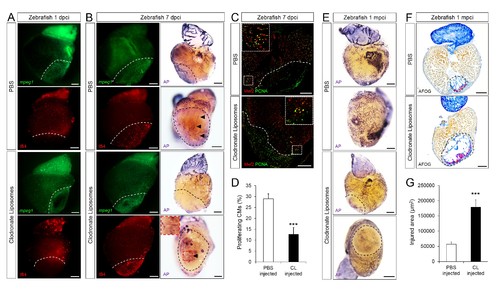
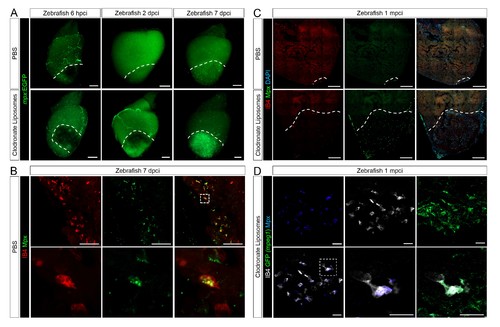
Delayed macrophage recruitment in zebrafish compromises neovascularization, CM proliferation and scar resolution. Adult zebrafish were injected with PBS or clodronate liposomes 1 day before injury. Hearts were collected at 1 dpci (A), 7 dpci (B and C) and 1 mpci (E–G) to examine macrophages by mpeg1:EGFP expression and IB4 staining (A and B), neovascularization by alkaline phosphatase (AP) staining (B and E, a side view as well as an apex view are shown), CM proliferation by PCNA/Mef2 immunostaining (C), and scar resolution by Acid Fuchsin Orange G (AFOG) staining (F). Mef2 and PCNA double positive CMs within 200 μm of the injured area were quantified in (D). Sections with the largest injured area/scar for each 1 mpci heart (delineated by black dotted lines) were stained with AFOG and quantified in G (n > 5, shown in Figure 4—figure supplement 2). Dotted lines delineate the injured area, arrowheads point to vessels, asterisks mark the initial injury site; scale bars, 200 μm. N ≥ 3 for each treatment and time point. Clodronate injections diminished macrophage recruitment at 1 dpci, and macrophage numbers recovered to control levels at 7 dpci (A and B, quantification in Figure 4—figure supplement 1 and Figure 4—figure supplement 1—source data 1). Delayed macrophage recruitment compromised neovascularization (B and E), and CM proliferation (C and D), and resulted in delayed scar resolution (F and G). |
|
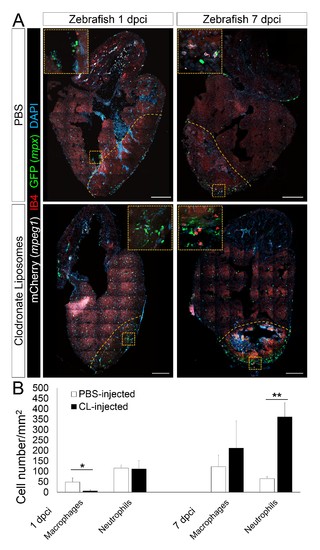
Delayed macrophage recruitment in zebrafish by Clodronate liposome pre-depletion. Adult mpeg1.4:mCherry-F;mpx:GFP zebrafish were injected with PBS or clodronate liposomes 1 day before injury. Hearts were collected at 7 dpci, and cryosections stained with GFP antibody to examine neutrophils, and mCherry antibody and IB4 to examine macrophages. Positive cells, both in the injured area itself and within 100 μm of the injured area, were quantified (B; n ≥ 3). Dotted lines delineate the injured area; scale bars, 200 μm. At 1 dpci, macrophage numbers in CL-exposed hearts were significantly lower than in controls, while neutrophils numbers were similar. At 7 dpci, neutrophil numbers were significantly higher in CL-exposed hearts, while macrophage numbers were not significantly different. |
|
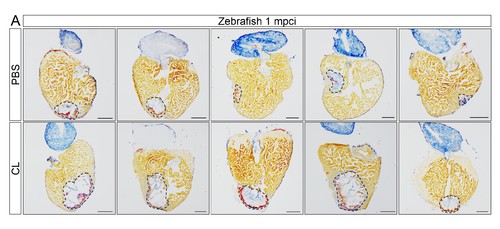
Delayed macrophage recruitment in zebrafish compromises scar resolution. Adult zebrafish were injected with PBS or clodronate liposomes 1 day before injury. Hearts were collected at 1 mpci, and cryosections were stained with AFOG. Healthy muscle stained in orange, fibrin in red, and collagen in blue. Sections with the largest scar area for each heart (delineated by dotted lines) were selected and quantified (Figure 4G, n = 5). Scale bars, 200 μm. Scar tissue in CL-exposed hearts was significantly larger than in controls at 1 mpci. |
|
Neutrophil clearance is delayed after macrophage pre-depletion. Adult zebrafish were injected with PBS or clodronate liposomes 1 day before injury. Hearts were collected at 6 hpci (A), 2 dpci (A), 7 dpci (A and B), and 1 mpci (C and D) to examine macrophages by both IB4 staining and mpeg1:EGFP expression, and neutrophils by immunostaining for both Mpx and mpx:GFP expression. (A) Neutrophil dynamics at 6 hpci, 2 and 7 dpci. (B) Neutrophil clearance by macrophage phagocytosis was observed in PBS-exposed hearts at 7 dpci. (C) Neutrophils remained in the injured area of CL-exposed hearts at 1 mpci. (D) Neutrophil clearance by IB4- and mpeg1:EGFP-double positive macrophages was observed in CL-exposed hearts at 1 mpci. Dotted lines delineate injured area; scale bars, 200 μm (A), 50 μm (B), 100 μm (C), and 20 μm (D). |
|
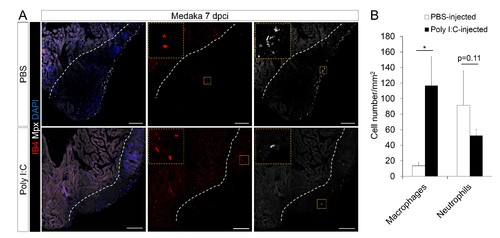
Poly I:C injection in medaka promotes macrophage recruitment and neutrophil clearance following cardiac injury. (A) Medaka heart sections at 7 dpci were stained with IB4 for macrophages and Mpx antibody for neutrophils. Positive cells, both in the injured area itself and within 100 μm of the injured area, were quantified (B; n = 3). Dotted lines delineate the injured area; scale bars, 100 μm. Poly I:C injection significantly promoted macrophage recruitment and neutrophil clearance in cryoinjured medaka hearts at 7 dpci. |
|
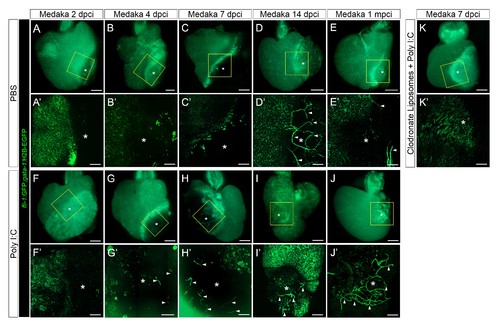
Poly I:C injection in medaka promotes neovascularization in a macrophage-dependent manner following cardiac injury. Adult fli::GFP;gata1::GFP medaka were injected with PBS (A–E) or poly I:C (F–J) immediately after injury, or with clodronate liposomes at 1 day before injury and poly I:C immediately after injury (K). Hearts were collected at 2 (A and F), 4 (B and G), 7 (C, H, and K), and 14 (D and I) dpci, as well as 1 mpci (E and J), and imaged with a stereo (A–K) or confocal (A'-K', boxed areas in A-K) microscope for detailed examination. Asterisks mark the injured area, and arrowheads point to new vessel-like structures. Initiation of vessel formation was observed at earlier time points (4 and 7 dpci) in poly I:C-exposed hearts compared to control hearts, and these vessels were maintained at least until 1 mpci (n > 3). In contrast, in control hearts, new vessels were observed only at 14 dpci, and largely disappeared by 1 mpci. In addition, vessel formation promoted by poly I:C injections was blocked by CL pre-injections. |
|
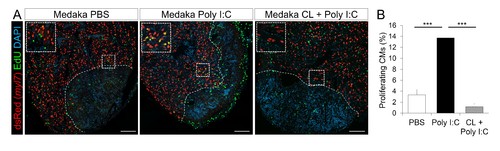
Poly I:C injection in medaka promotes CM proliferation in a macrophage-dependent manner following cardiac injury. Adult zfmlc2 5.1 k:DsRED2-nuc medaka were injected with PBS or poly I:C immediately after injury, or with clodronate liposomes at 1 day before injury and poly I:C immediately after injury. Hearts were collected at 7 dpci, and CM proliferation was examined by EdU labeling and immunostaining for DsRED expression; dotted lines delineate the injured area. EdU positive CMs within 200 μm of the injured area were quantified and shown (B; n ≥ 3). Scale bars, 100 μm. The percentage of proliferating CMs was significantly higher in poly I:C-exposed hearts compared to both PBS- and CL + poly I:C-exposed hearts. |
|
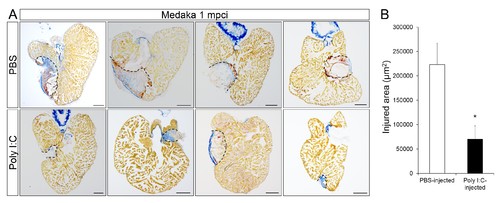
Poly I:C injection in medaka promotes scar resolution following cardiac injury. Adult fli::GFP;gata1::GFP medaka were injected with PBS or poly I:C immediately after injury. Hearts were collected at 1 mpci, and scar composition and resolution were examined by AFOG staining. (A) Healthy muscle stained in orange, fibrin in red, and collagen in blue. Sections with the largest scar area for each heart (delineated by dotted lines) were selected and quantified (B; n ≥ 4). Scale bars, 200 μm. Scar tissue in poly I:C-exposed hearts was significantly smaller than in controls at 1 mpci. |