- Title
-
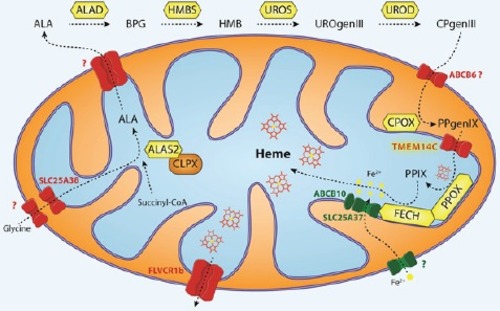
Mitochondrial transport of protoporphyrinogen IX in erythroid cells
- Authors
- Yien, Y.Y., Ringel, A.R., Paw, B.H.
- Source
- Full text @ Oncotarget
|
Glycine is imported via SLC25A38 and condenses with succinyl-CoA to form δ-aminolevulinic acid (ALA) in a reaction catalyzed by ALA synthase (ALAS2 in red cells) [ |