- Title
-
Wnt signaling balances specification of the cardiac and pharyngeal muscle fields
- Authors
- Mandal, A., Holowiecki, A., Song, Y.C., Waxman, J.S.
- Source
- Full text @ Mech. Dev.
|
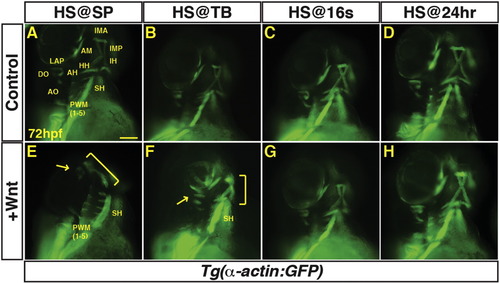
Excess Wnt signaling inhibits PM development during gastrulation. (A–P) Tg(α-actin:GFP) control sibling embryos and embryos with increased Wnt signaling (hsp70l:wnt8a-GFP+). (A, E) After increasing Wnt signaling from heat-shock (HS) at the SP stage, the EOMs, dorsal 1st and 2nd arch PMs are lost (arrow), while the ventral 1st and 2nd arch PMs are reduced (bracket). PW muscles are also reduced, while the SH was overtly unaffected. (B, F) After increasing Wnt signaling at the TB stage, there is a similar but less severe loss of EOMs, 1st and 2nd arch PMs (dorsal PMs – arrow; ventral PMs bracket). (C, G, D, H) Wnt signaling does not overtly affect PM development at later stages. Muscle nomenclature used is from ( Schilling and Kimmel, 1997). 1st arch muscles - intermandibularis anterior (IMA), intermandibularis posterior (IMP), adductor mandibulae (AM), levator arcus palatine (LAP), dilatator opercula (DO). 2nd (hyoid) arch muscles - hyohyal (HH), interhyal (IH), adductor hyomandibulae (AH), adductor opercula (AO), and levator opercula (LO). Embryos are at 72 hpf. Images are anterior ventro-lateral views. Anterior is up in all images. Heat-shock (HS). > 15 embryos were examined for each condition. Scale bar indicates 100 μm. |
|
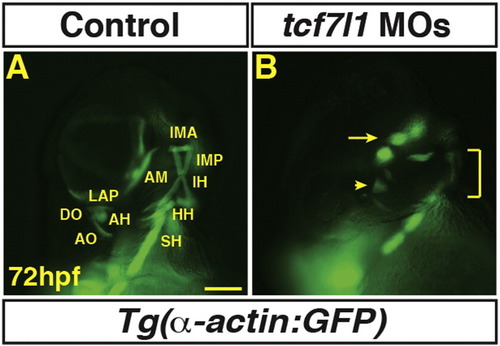
Tcf7l1 depletion causes loss of dorso-anterior PMs. (A–B) Control and Tcf7l1 depleted Tg(α-actin:GFP) embryos at 72 hpf. Tcf7l1 depletion resulted in similar loss of dorso-anterior PMs as increasing Wnt signaling prior to gastrulation. EOMs (arrow), 1st and 2nd PMs are severely reduced and misorganized or lost (arrowhead). Ventral 1st and 2nd arch muscles are reduced (bracket). Views are anterior ventro-lateral. > 15 embryos were examined for each condition. Scale bar indicates 100 μm. |
|
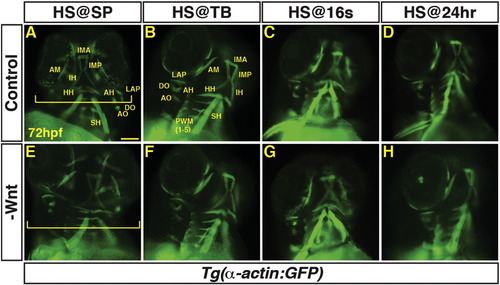
Decreased Wnt signaling during gastrulation increases head size with minimal effect on PM morphology. (A–H) Tg(α-actin:GFP) control sibling embryos and embryos with decreased Wnt signaling (hsp70l:dkk1-GFP+). (A, E) After decreasing Wnt signaling at the SP stage, the embryos have enlarged heads (brackets). (B–D, F–H) After decreasing Wnt signaling at later stages, there was no discernible effect on pharyngeal muscles. Embryos are at 72 hpf. Images in A,E are anterior ventral views. Images in B–D, F–H are anterior ventro-lateral views. Anterior is up in all images. Heat-shock (HS). > 15 embryos were examined for each condition. Scale bar indicates 100 μm. |
|
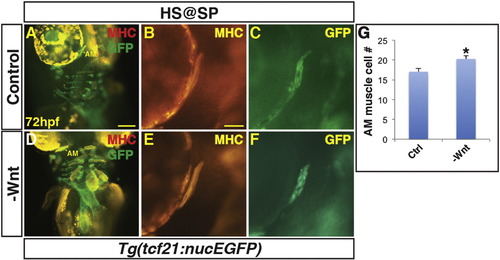
Decreased Wnt signaling causes an increase in 1st arch PM cells. (A, B) Tg(tcf21:nucEGFP) control sibling embryos and embryos with decreased Wnt signaling at the SP stage. Decreased Wnt signaling causes enlargement of the 1st arch AM muscle (boxes). Sarcomeric myosin (MHC; red). NucGFP (green). (B, E) MHC alone of AM muscles. (C, F) NucEGFP alone of AM. (G) Graph depicting quantification of nuclei in the AM muscles. Decreasing Wnt signaling at the SP stage produces a modest, but significant increase in AM muscle nuclei. Control embryos n = 15, Tg(hsp70l:dkk1-GFP) embryos n = 7. Asterisks in all graphs indicate p < 0.05. Error bars in all graphs indicate S.E.M. Scale bar in A indicates 100 μm. Scale bar in B indicates 50 μm. |
|
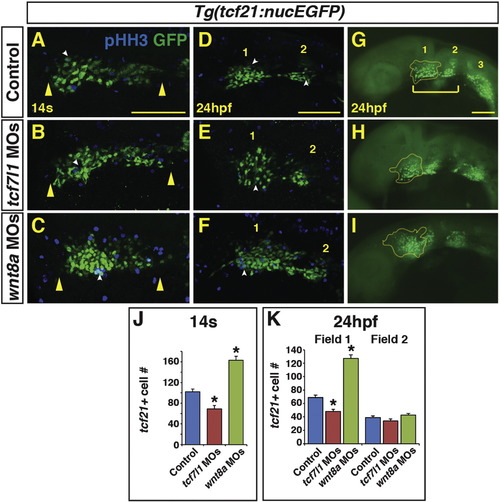
Endogenous Wnt signaling components restrict tcf21+ PM progenitor development. (A-C) tcf21+ (GFP+) cells within the ALPM of Tg(tcf21:nucEGFP) control, Tcf7l1 depleted, and Wnt8a depleted embryos at the 14 s stage. Images are dorsal views of the left side of the embryos with anterior left. Yellow arrowheads indicate size of field. (D–I) tcf21+ (GFP+) cells within the ALPM of Tg(tcf21:nucEGFP) control, Tcf7l1 depleted, and Wnt8a depleted embryos at 24 hpf. Images are lateral views with anterior left. (E, H) Tcf7l1 depletion produces a loss of the anterior most tcf21+ progenitors (yellow outline). (F, I) Wnt8a depletion results in expansion of the anterior most pharyngeal tcf21+ progenitors. White arrowheads in A–F indicate overlap between GFP+ and phospho-histone H3 (pHH3)+ cells (see Supplementary material – Fig. S3 for analysis of pHH3). Bracket in G indicates the anterior tcf21+ field of cells that give rise to the 1st and 2nd arch muscles. Numbers 1 and 2 in D-G indicate the two anterior tcf21+ fields of cells. Number 3 in G indicates the more posterior tcf21+ population that gives rise to PM in arches 3–7 ( Nagelberg et al., 2015). Images A–F are confocal images. (J) Graph depicting quantification of tcf21+ progenitors at the 14 s stage. Tg(tcf21:nucEGFP) control n = 15, Tcf7l1 depleted n = 10, and Wnt8a depleted embryos n = 10. (K) Graph depicting quantification of tcf21+ progenitors at the 24hpf. Tg(tcf21:nucEGFP) control n = 10, Tcf7l1 depleted n = 13, and Wnt8a depleted embryos n = 10. Cell counts are from confocal images of the tcf21+ field on one side of the embryo. There is a significant decrease in tcf21+ progenitors with Tcf7l1 depletion and conversely a significant increase in tcf21+ progenitors following Wnt8a depletion in the total field at the 14 s stage and anterior field at 24 hpf. Scale bars indicates 100 μm. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.) |
|
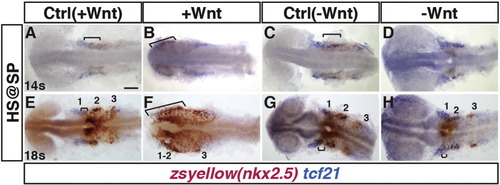
Manipulation of Wnt signaling affects the overlap of nkx2.5+ and tcf21+ domains within the ALPM. (A–H) Tg(nkx2.5:ZsYellow) control sibling embryos and embryos with increased and decreased Wnt signaling at the SP stage. (A, B, E, F) Two-color ISH of zsyellow (nkx2.5; brownish red) and tcf21 (blue) at the 14 s and 18 s stages shows expansion of the lateral nkx2.5+ domains into the anterior tcf21+ domain after increased Wnt signaling. Numbers in E indicate the three tcf21+ fields depicted in Fig. 5. (C, D, G, H) Decreasing Wnt signaling prior to gastrulation causes a decrease in the overlap of nkx2.5 and tcf21 at the 14 s and 18 s stages. Significant overlap between nkx2.5 and tcf21 could not be detected at the 14 s stage in embryos when with Wnt signaling was inhibited prior to gastrulation. Images are dorsal views with anterior to left. Brackets indicate overlap of nkx2.5+ and tcf21+ domains. For each group > 15 embryos were examined. Scale bar indicates 100 μm. |
|
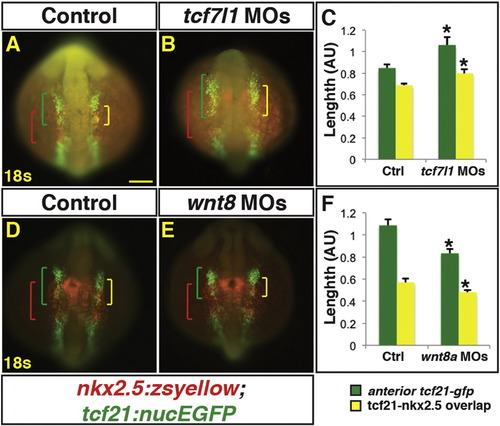
Manipulation of endogenous Wnt signaling affects the size of the nkx2.5+ and tcf21+ domains. (A, B, D, E) Tg(nkx2.5:ZsYellow);Tg(tcf21:nucEGFP) control, Tcf7l1 depleted, and Wnt8a depleted embryos at the 18 s stage. Tcf7l1 depletion expands the total anterior tcf21+ progenitor domain length (green brackets) as well as the overlapping expression (yellow brackets) within the ALPM. (C) Graph depicting measurements of the total tcf21+ progenitors and overlap with nkx2.5+ progenitors from Tcf7l1 depleted embryos. Control embryos n = 10, Tcf7l1 depleted embryos n = 9. (D, E) Wnt8a depletion reduces the overall length of the tcf21+ domain and the overlap with the nkx2.5+ progenitor domain. (F) Graph depicting measurements of the total tcf21+ progenitors and overlap with nkx2.5+ progenitors from Wnt8a depleted embryos. Control embryos n = 8, Wnt8a depleted embryos n = 10. Green brackets - anterior tcf21+ progenitors. Red brackets - nkx2.5+ progenitors. Yellow brackets – regions of overlap between tcf21+ and nkx2.5+ domains. Scale bar indicates 100 μm. |
|
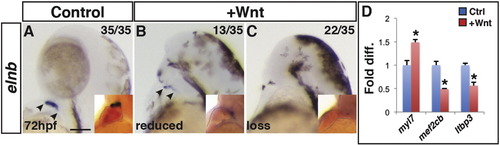
Increased Wnt signaling promotes loss of the SHF. (A–C) Control sibling embryos and embryos after increased Wnt signaling at the SP stage. Increasing Wnt signaling prior to gastrulation leads to loss of the SHF-derived OFT smooth muscle (elnb). Inserts indicate embryos with two-color ISH for myl7 and elnb. Outlines indicate the hearts. (D) RT-qPCR for cardiac differentiation and SHF progenitor marker expression at 24 hpf. Increasing Wnt signaling prior to gastrulation promotes increased expression of the pan-cardiac differentiation marker myl7 and decreased expression of the SHF markers mef2cb and ltbp3 compared to WT sibling controls. Images are lateral views with anterior upward. Scale bar indicates 100 μm. |
|
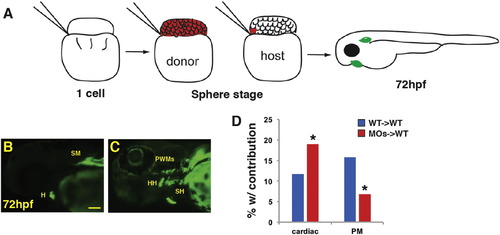
Wnt signaling cell-autonomously inhibits PM specification. (A) Schematic of blastula cell transplantation method. (B, C) Representative donor cells that incorporated into the heart and PMs at 72 hpf. (D) Graph indicating the frequency of donor cell contribution in embryos to the heart and the PMs. WT donor cells → WT host transplants n = 240. Tcf7l depleted donor cells → WT host embryos n = 221. Tcf7l1 depleted donor cells contributed significantly more to the cardiomyocytes and less frequently to the PMs compared to WT donors. Images are lateral views with anterior leftwards. Scale bar indicates 100 μm. |
|
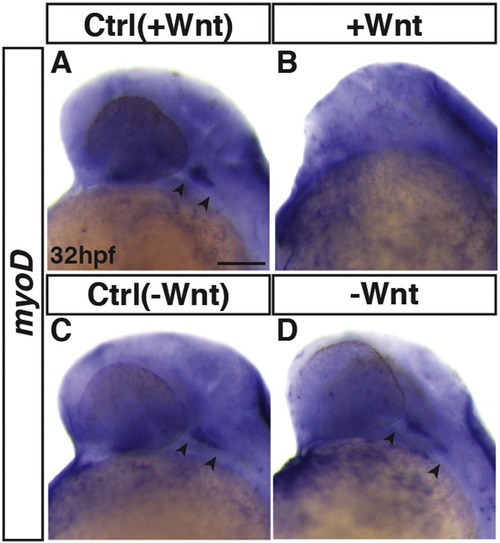
Manipulation of Wnt signaling affects cranial myod expression. ISH for myod at 32 hpf. (A, B) Control sibling embryos and embryos with increased Wnt signaling (hsp70l:wnt8a-GFP+). (C, D) Control sibling embryos and embryos with decreased Wnt signaling (hsp70l:dkk1-GFP+). Arrows indicate length of expression in the head. No expression was observed in embryos with excess Wnt signaling at 32 hpf. ≥ 10 embryos were examined per condition. Anterior is to the left. Scale bar indicates 100 μm. |
|
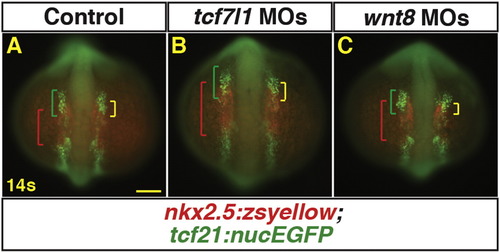
Manipulation of Wnt signaling affects the overlap of cardiac and pharyngeal muscle progenitor markers. (A–C) Tg(nkx2.5:ZsYellow);Tg(tcf21:nucEGFP) control, Tcf7l1 depleted, and Wnt8a depleted embryos at the 14 s stage. Tcf7l1 depletion expands the total anterior tcf21 + progenitor domain length (green brackets) as well as the overlapping expression (yellow brackets) within the ALPM. Green brackets - anterior tcf21+ progenitors. Red brackets - nkx2.5+ progenitors. ≥ 5 embryos were examined per condition. Yellow brackets – regions of overlap between tcf21+ and nkx2.5+ domains. Scale bar indicates 100 μm. |
|
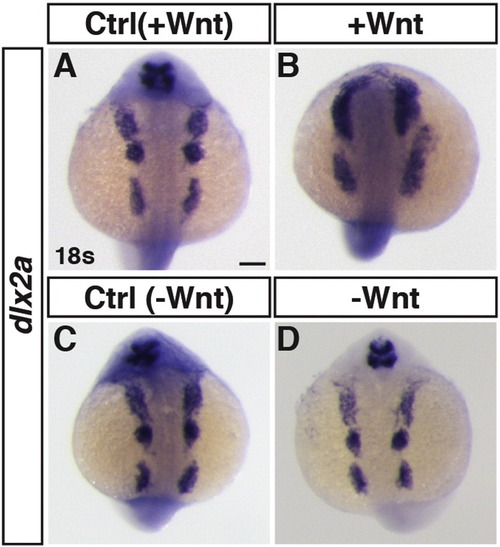
Effects of early Wnt signaling modulation on neural crest marker expression. ISH for dlx2a at 18 s stage. (A,B) Control sibling embryos and embryos with increased Wnt signaling (hsp70l:wnt8a-GFP+). The anterior 1st and 2nd neural crest streams are fused and the 3rd is expanded, consistent with posteriorization of the embryo. (C,D) Control sibling embryos and embryos with decreased Wnt signaling (hsp70l:dkk1-GFP+). The neural crest cells were minimally affected after loss of Wnt signaling. Images are dorsal views. Anterior is up. ≥ 15 embryos were examined per condition. Scale bar indicates 100 μm. |
|
Effects of early Wnt signaling modulation on cranial cartilage. Alcian blue staining at 72 hpf. (A, B) Control sibling embryos and embryos with increased Wnt signaling (hsp70l:wnt8a-GFP+). The anterior neural crest-derived cartilaginous arches are lost and malformed after increased Wnt signaling. (C,D) Control sibling embryos and embryos with decreased Wnt signaling (hsp70l:dkk1-GFP+). The neural crest derived cartilage was minimally affected after loss of Wnt signaling, despite the larger heads. Images are anterior and ventral views. Anterior is up. ≥ 15 embryos were examined per condition. Scale bar indicates 100 μm. |
|
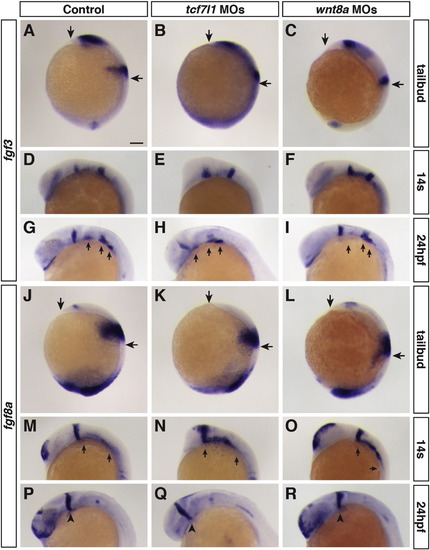
Consequences of manipulation of early Wnt signaling on Fgf ligands. (A–I) Fgf3 expression in control, Tcf7l1 depleted, and Wnt8 depleted embryos at the designated stages. (J–R) Fgf8a expression in control, Tcf7l1 depleted, and Wnt8 depleted embryos at the designated stages. Changes in expression domains are consistent with effects of early Wnt signaling on anterior-posterior patterning. Arrows in A–C and J–L mark the anterior head and posterior limit of expression for the markers in the presumptive midbrain-hindbrain boundary. Arrows in G–I indicate expression in the pharyngeal endodermal pouches. Fgf3 expression in the pouches is not affected by manipulation of early Wnt signaling. Arrows in M-O indicate expression of fgf8a in the ALPM. Arrows in P-R indicated midbrain-hindbrain boundary expression of fgf8a. ≥ 10 embryos were analyzed for each condition. Scale bar indicates 100 μm. |
|
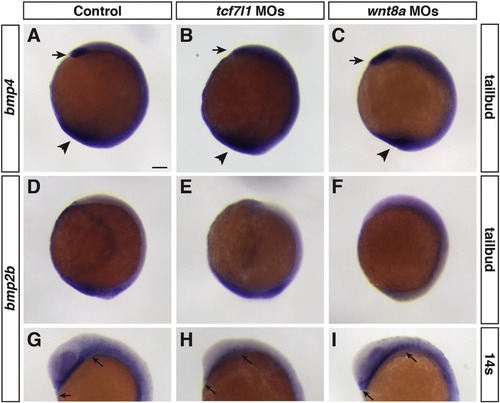
Consequences of manipulation of early Wnt signaling on Bmp ligands. (A–C) Bmp4 expression in control, Tcf7l1 depleted, and Wnt8 depleted embryos at the tailbud stage. Arrows indicate anterior expression. Arrowhead indicates ventral-posterior expression. (D–I) Bmp2b expression in control, Tcf7l1 depleted, and Wnt8 depleted embryos at the tailbud and 14 s stages. Changes in bmp expression domains are consistent with effects of early Wnt signaling on anterior-posterior patterning. Arrows in G-I mark the anterior endoderm, which is reduced but not completely in Tcf7l1 depleted embryos. ≥ 10 embryos were analyzed for each condition. Scale bar indicates 100 μm. |
Reprinted from Mechanisms of Development, 143, Mandal, A., Holowiecki, A., Song, Y.C., Waxman, J.S., Wnt signaling balances specification of the cardiac and pharyngeal muscle fields, 32-41, Copyright (2017) with permission from Elsevier. Full text @ Mech. Dev.