- Title
-
ELMO1 protects renal structure and ultrafiltration in kidney development and under diabetic conditions
- Authors
- Sharma, K.R., Heckler, K., Stoll, S.J., Hillebrands, J.L., Kynast, K., Herpel, E., Porubsky, S., Elger, M., Hadaschik, B., Bieback, K., Hammes, H.P., Nawroth, P.P., Kroll, J.
- Source
- Full text @ Sci. Rep.
|
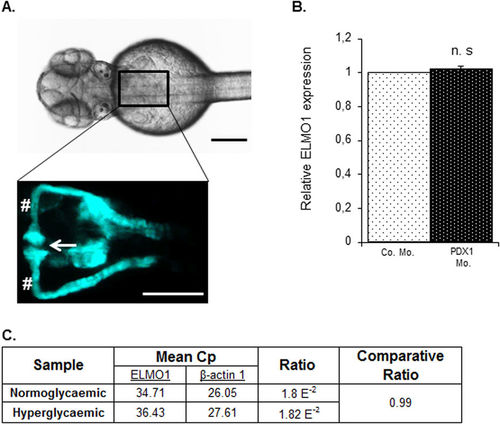
Expression of ELMO1 is observed within the zebrafish pronephros but remains unaltered under hyperglycaemic conditions as compared to normoglycaemic fish. (A) Illustrates the position of the pronephros in a Tg(wt1b:EGFP) embryo (48 hpf), visible from the dorsal view. The renal structure is composed of two tubules that are fused to form a single glomerulus (white arrow) via a neck (white hashtag). Both the scale bars represent 200 μm. (B) No change in relative ELMO1 expression was observed within the zebrafish pronephros under hyperglycaemic conditions (4 ng morpholino injection against insulin promotor factor 1, PDX1). Co. Mo. indicates 4 ng control morpholino. Pronephric cells were isolated for mRNA extraction via FACS sorting from Tg(wt1b:EGFP) embryos for cDNA synthesis and subsequent real-time qPCR analysis. (C) tabulates the mean Cp (crossing point of the amplifying fluorescence exceeding the background fluorescence) values of ELMO1 (target) and β-actin 1 (reference) expression. EXPRESSION / LABELING:
|
|
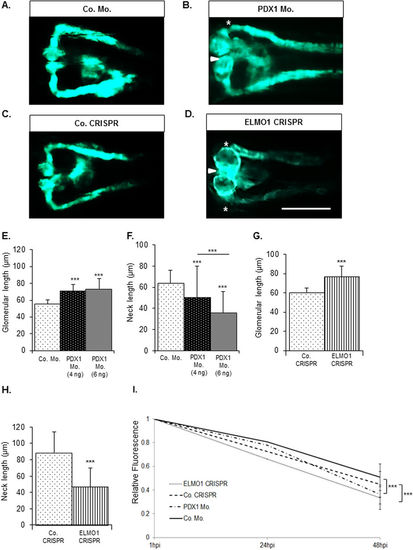
Zebrafish pronephric structure and function are adversely affected by hyperglycaemia and upon CRISPR mediated ELMO1 knockout. As compared to the zebrafish pronephros at 48 hpf in control morphants, Co. Mo., (A) hyperglycaemic PDX1 morphants, PDX1 Mo., (B) display an enlarged glomerulus (white arrow head) and highly shortened pronephric neck (white asterix). (C) Control crispanst (Co. CRISPR) display a normal glomerulus. (D) In contrast, ELMO1 crispants, (ELMO1 CRISPR), show an enlarged glomerulus (white arrow head) and a highly shortened pronephric neck (white asterix). (E–H) The altered structure in morphants and crispants has been quantified in n = 40 embryos for each condition. (I) Displays the quantified elevated loss of fluorescence in ELMO1 crispants (n = 38) and PDX1 morphants (n = 26) as compared to their respective controls (Co. CRISPR, n = 39 and Co. Mo., n = 30). The white line in image (D) represents the scale bar corresponding to 200 μm. |
|
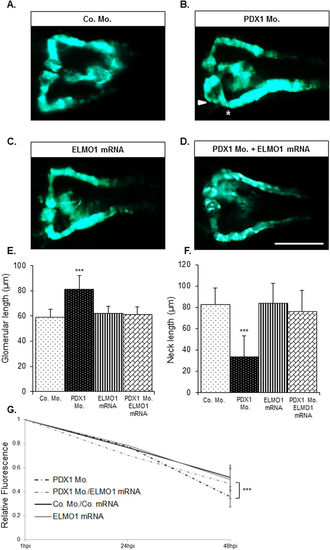
Over-expression of ELMO1 rescues the renal structural and functional phenotype caused due to hyperglycaemia. As compared to the zebrafish pronephros at 48 hpf in control morphants, Co. Mo., (A) hyperglycaemic PDX1 morphants, PDX1 Mo. (4 ng), (B) display an enlarged glomerulus (white arrow head) and a highly shortened pronephric neck (white asterix). (C) Overexpression of ELMO1 has no detrimental effects on the zebrafish pronephros, however, (D) overexpression of ELMO1 in PDX1 morphants significantly rescues pronephric phenotype. (E,F) The altered structure in PDX1 morphants and their subsequent rescue with ELMO1 overexpression is quantified in n = 40 embryos, for each condition. (G) Displays the quantified elevated loss of fluorescence in PDX1 morphants (n = 23) and the significant increase in fluorescence within hyperglycaemic fish upon ELMO1 overexpression (Double Co. Mo. and mRNA, n = 23; ELMO1 mRNA, n = 23; and PDX1 Mo. with ELMO1 mRNA, n = 23). |
|
Knockdown of PDX1 and knockout of ELMO1 causes alterations in podocytic structure within zebrafish embryos aged 48 hpf. (A,B) Podocytes (P) in Co. Mo. and Co. CRISPR zebrafish embryos aged 48 hpf are well developed and have well defined foot processes that interdigitate amongst themselves to form functional filtration barriers (white arrow heads). (C) Upon PDX1 knockdown, complete distrophy of podocyte foot processes is observed. (D) Upon ELMO1 knockout, foot processes are immature and are unable to interdigitate to form an appropriate filteration barrier. The white line indicates the scale bar corresponding to 1 μm length. Number of embryos analysed per sample group were 10. PHENOTYPE:
|
|
Compromised pronephric structure and ultrafiltration in ELMO1 crispants, but not in PDX1 morphants, can be beneficially reversed upon treatment with pancaspase inhibitor, zVAD-fmk. Upon treatment with zVAD-fmk (300 μM), pronephric structural defects in ELMO1 crispants at 48 hpf, are significantly restored to normalcy (A,B,D,E); n = 47 for each condition). The pancaspase inhibitor causes no adversity in the control embryos at 48 hpf (D). However, no significant restoration in pronephric structure is observed with zVAD-fmk treatment of PDX1 morphants, at the same age (C,F); n = 47 for both conditions). The white line in image (F) represents the scale bar corresponding to 200 μm. The significant restoration of the glomerular width and neck via zVAD-fmk treatment in ELMO1 crispants as well as the lack of rescue in PDX1 morphants are quantified in graphs (G,H). Altered ultrafiltration in ELMO1 crispants is also improved significantly upon treatment with zVAD-fmk as seen in graph (I) but not in PDX1 morphants, as observed in (J) The n values for each sample set used for the functional assay are as follows: Co. CRISPR (22), Co. CRISPR with zVAD-fmk (23), ELMO1 CRISPR (22), ELMO1 CRISPR with zVAD-fmk (17), Co. Mo. (23), Co. Mo. with zVAD-fmk (23), PDX1 Mo. (26) and PDX1 Mo. with zVAD-fmk (22). |
|
ELMO1 knockout, but not hyperglycaemia, causes an increase in apoptotic cells within the zebrafish embryo as well as the pronephros, which can be rescued with pancaspase inhibitor, zVAD-fmk. (A) TUNEL assay was carried out on Co. CRISPR injected Tg(wt1b:EGFP) embryos at 48 hpf. The embryos showed no incidence of apoptosizing cells (indicated with the red spots) within the renal structure. (B) ELMO1 CRISPR injected embryos showed a strong incidence of apoptosizing cells (red spots) universally as well as within the renal structure. (C) Indicated that hyperglycaemic embryos do not display increased apoptosis within the pronephros and (D) indicates that ELMO1 crispants displayed normalized renal structure and a drastic reduction of apoptosis, upon zVAD-fmk treatment, which is quantified in the graph. (E) The white line in (D) indicates a scale bar representing 100 μm, the white arrow head indicated an enlarged glomerulus and the white asterisk illustrates shortening of the glomerular neck of the ELMO1 crispants as compared to the control. The white dotted circle encloses the glomerulus of the zebrafish pronephros. The number of embryos per condition analysed are 11. |
|
Measurement of heart fluorescence over time for the pronephric functional assesment in zebrafish. Texas Red® tagged 70 kDa dextran was injected into the heart of ELMO1 mosaic mutants, PDX1 morphants and their respective controls, in zebrafish embryos, aged 72 hpf. Images were taken at 1 hpi, 24 hpi and 48 hpi and the relative fluorescence in the heart region, highlighted in red, was measured using imageJ. A. indicates the development and loss of fluorescence in an ELMO1 mosaic mutant over indicated time. The white line represents a scale bar of 200 μm. |
|
Pronephric structural alterations in ELMO1 crispants could be rescued completely via ELMO1 mRNA. As compared to the zebrafish pronephros at 48 hpf in controls, Co. CRISPR + mRNA., A., ELMO1 over-expression B., has no detrimental effects on the zebrafish pronephros. C. ELMO1 crispants display detrimental effects on the zebrafish pronephric structure, with an enlarged glomerulus (white arrow head) and highly shortened pronephric neck (white asterix). However, D., overexpression of ELMO1 in ELMO1 crispants significantly rescues pronephric phenotype. E., F. the altered structure in ELMO1 crispants and their subsequent rescue with ELMO1 overexpression is quantified in n=40 embryos, for each condition. The white line in D., indicates a scale bar of 200 μm. |
|
CRISPR mediated knockout of ELMO1 causes an increase in apoptotic cells within the zebrafish embryo as well as the pronephros. A. Activated Caspase 3 assay was carried out on Co. CRISPR injected Tg(wt1b:EGFP) embryos at 48 hpf. The embryos showed no incidence of apoptosizing cells (indicated with the red spots) within the renal structure. B. ELMO1 CRISPR injected embryos showed a strong incidence of apoptosizing cells (red spots) universally as well as within the renal structure, which is quantified in the graph C. The number of embryos per condition analysed are 11. The white line indicates a scale bar representing 100 μm, the white arrow head indicated an enlarged glomerulus and the white asterisk illustrates shortening of the glomerular neck of the ELMO1 crispants as compared to the control. The white dotted circle encloses the glomerulus of the zebrafish pronephros. |