- Title
-
The tbx2a/b transcription factors direct pronephros segmentation and corpuscle of Stannius formation in zebrafish
- Authors
- Drummond, B.E., Li, Y., Marra, A.N., Cheng, C.N., Wingert, R.A.
- Source
- Full text @ Dev. Biol.
|
tbx2a/b transcripts are specifically expressed in the caudal domain of the renal progenitor field during nephrogenesis. (A) Schematic of zebrafish pronephros development. The intermediate mesoderm (IM) gives rise to the two-nephron pronephros, which is fully segmented by 24 hpf (28 ss): P (podocytes), N (neck), PCT (proximal convoluted tubule), PST (proximal straight tubule), DE (distal early tubule), CS (corpuscle of Stannius), DL (distal late tubule) and PD (pronephric duct). (B) Whole mount in situ hybridization shows that tbx2a and tbx2b transcripts are expressed in the distal region of the pronephros. At 14 ss, tbx2a transcripts can be detected in the distal region of the intermediate mesoderm while tbx2b transcripts are observed in a smaller distal domain. At 22 ss, tbx2b expression in the distal pronephros closely resembles tbx2a expression in this region. However, by 28 ss, tbx2a transcripts are most intensely expressed in the PD segment and trail off in the DL segment, while tbx2b transcripts are evident throughout the DL and more sparsely in the PD. (C) Flat-mount images of embryos co-stained with tbx2a and myoD, and tbx2b and myoD show that tbx2a is more widely expressed in the emergent distal pronephros than tbx2b at 14 ss. At 16 ss, tbx2b expression expands and begins to resemble tbx2a expression. (D) Fluorescent in situ hybridization shows that tbx2a and tbx2b are co-expressed in portions of the PD and DL at 28 ss. Specifically, tbx2a is expressed more intensely in the PD than tbx2b. In addition, tbx2b has a stronger expression in the DL, while tbx2a expression begins to taper off. EXPRESSION / LABELING:
|
|
tbx2a/b exhibit co-expression with the DL and CS. (A) Fluorescent in situ hybridization was used to examine the distal region of the pronephros of wild-type 28 ss embryos, with DAPI (blue) used to label nuclei. tbx2a expression again appears strongest in the PD segment and fades as it progresses anteriorly. The region in the white box represents the moderate co-expression seen between tbx2a and slc12a3 near the boundary of the slc12a3 domain. In contrast, tbx2b expression appears strongly co-localized through most of the length of the DL, as exemplified by the region in the white box. In addition, tbx2b continues to be expressed past the DL in the PD segment. (B) The transcription factor sim1a, which has been demonstrated to be a necessary requirement for CS formation, shows specific co-localization with CS marker stc1 in wild-type, 28 ss embryos. This indicates that sim1a can be used as a secondary indicator for changes in the CS. Additionally, when examining the CS in wild-type embryos at 28 ss it was noted that tbx2a is not expressed in or near the CS while tbx2b does co-localize with stc1 in the CS at this stage. EXPRESSION / LABELING:
|
|
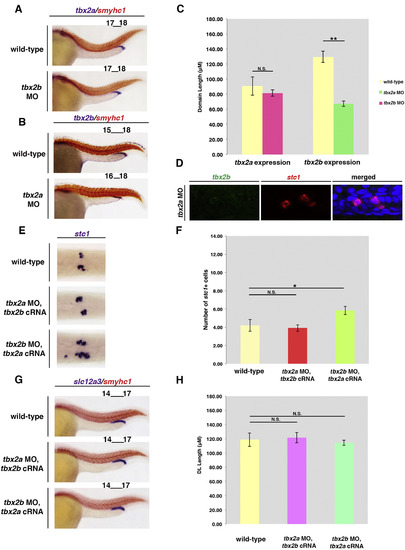
tbx2a/b deficiency leads to a decrease in the size of the DL segment and a slight expansion in the PCT segment in the pronephros. Upper panel: At 28 ss, double whole mount in situ hybridization was used to label segmental domains in wild-type and tbx2a/b deficient embryos. Somites were labeled using smyhc1 (red) to detect length changes in adjacent pronephros segments (purple). Within proximal domains, MO knockdown of tbx2a, tbx2b, or tbx2a/b induced a 1-somite expansion in the slc20a1a-expressing PCT segment in morphant pronephros. This same expansion was also seen in fbyc144 mutants (tbx2b deficient) and fbyc144 mutants injected with tbx2a MO. Within distal domains, tbx2a/b deficient morphants and mutants exhibited a 1–2 somite reduction in the slc12a3-expressing DL segment at 24 hpf. The PST and DE segments, labeled by transcripts encoding trpm7 and slc12a1, respectively, exhibited a 1 somite posterior shift in tbx2a/b deficient embryos compared to wild-type embryos due to changes in the domain size of the PCT and DL segments. Representative wild-type and morphant results are based on somite counting of at least 20 embryos per marker. In fbyc144 embryos, segments showed consistent changes based on somite counting of at least 3 genotype-confirmed mutants per marker. Lower panel: Schematic summary of pronephros segment organization in wild-type and tbx2a/b deficient embryos. Abbreviations: PCT (proximal convoluted tubule), PST (proximal straight tubule), DE (distal early tubule), DL (distal late tubule) and MO (morpholino). EXPRESSION / LABELING:
PHENOTYPE:
|
|
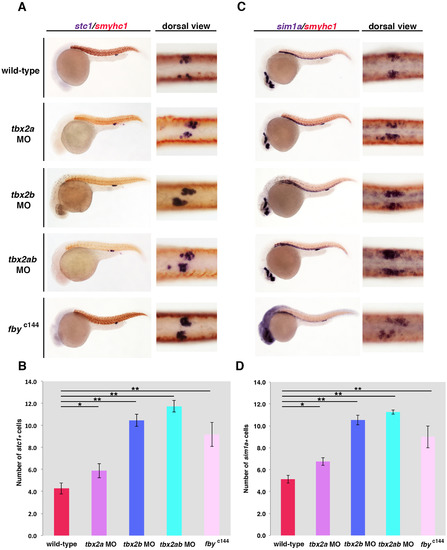
tbx2a/b deficiency leads to an increase in the number of cells in the CS. (A) The CS was labeled by whole mount in situ hybridization to detect transcripts encoding stc1 at the 28 ss stage. (B) In wild-type embryos, each CS is typically comprised of about 4–6 stc1+ cells per nephron. However, tbx2a/b deficiency in morphants and fbyc144 mutants induced a significant increase of the stc1+ cell number in the CS. (C) Labeling the CS with sim1a revealed a similar phenotype consistent with an inhibitory role of tbx2a/b in CS development. (D) Quantification of sim1a+ CS cell number revealed that tbx2a, tbx2b and tbx2a/b morphants and fbyc144 mutants all led to a significantly greater number of CS cells than wild-type embryos. For each experiment, at least 15 morphant embryos and 3 genotypically confirmed fbyc144 mutant embryos were examined. (*p<0.05, **p<0.001). Abbreviations: CS (corpuscle of Stannius). EXPRESSION / LABELING:
PHENOTYPE:
|
|
tbx2a/b are necessary and sufficient to promote the formation of the DL. (A) Injecting wild-type embryos with either tbx2a or tbx2b cRNA resulted in a significant expansion of the DL as observed by somite counting (smyhc1). (B) In wild-type, 28 ss embryos, the DL (slc12a3) domain and the DE (slc12a1) domain are distinctive. In embryos overexpressing either tbx2a or tbx2b, DE cells and DL cells appear to be co-mingled adjacent to the 13th somite. This observation explains why the DL expands to the 13th somite in tbx2a/b overexpressing embryos but the DE, PST, and PCT domains remain unaltered. DAPI (blue) was used to label nuclei (C) Rescue studies reveal that DL domain changes in tbx2a and tbx2b morphants are specific to loss of tbx2a and tbx2b, rather than toxicity. Wild-type embryos were co-injected with either tbx2a cRNA and tbx2a ATG MO or tbx2b cRNA and tbx2b ATG MO. In addition, fbyc144 mutants were injected with tbx2b cRNA. In all treatments, a wild-type DL was observed some of the time as indicated by somite counting and length measurements. (D) The DL of 5 embryos from each overexpression and rescue treatment was measured in uM. A significant increase in the DL was seen in animals injected with tbx2a cRNA and tbx2b cRNA, but no significant change in DL length was observed in any of the rescue experimental parameters. (*p<0.05, **p<0.001, N.S. = not significant). Abbreviations: cRNA (capped RNA), MO (morpholino), DL (distal late). |
|
tbx2a/b are necessary and sufficient to inhibit CS cellular expansion. (A) Dorsal images of wild-type 28 ss embryos injected with tbx2a and tbx2b cRNA show that tbx2a/b overexpression results in a decrease in the number of stc1-expressing CS cells. Rescue studies were conducted by co-injecting wild-type with either tbx2a cRNA and tbx2a MO or tbx2b cRNA and tbx2b MO and fbyc144 mutants with tbx2b cRNA. In all three conditions, the CS was successfully rescued. (B) The number of CS cells in each nephron was counted for at least 10 representative embryos from each condition. The number of CS cells in embryos injected with tbx2a cRNA and tbx2b cRNA were significantly lower than wild-type embryos. In contrast, the number of CS cells in rescue study embryos was not significantly different than wild-type embryos. (*p<0.05, **p<0.001, N.S.=not significant). Abbreviations: cRNA (capped RNA), CS (corpuscle of Stannius). |
|
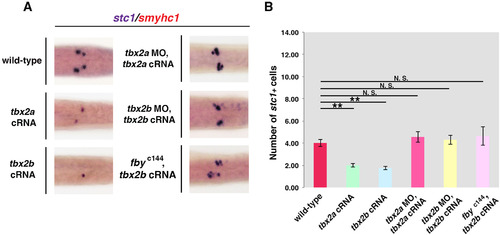
tbx2a functions upstream of tbx2b during pronephros segment and CS development. (A, B) Whole mount in situ hybridization was performed with tbx2a probe on 28 ss wild-type embryos and embryos injected with tbx2b MO. The tbx2a domain remained the same somite length despite the loss of tbx2b. In contrast, loss of tbx2a caused a notable decrease in the tbx2b domain. (C) The length of tbx2a and tbx2b expression domains of five representative embryos was measured (uM). While tbx2a expression is not significantly different wild-type and tbx2b morphants, the size of the tbx2b domain is significantly smaller than their wild-type siblings. (D) Fluorescent in situ hybridization was used to examine the distal region of the pronephros of tbx2a morphant embryos at the 28 ss, which revealed that stc1+ CS cells lacked tbx2b transcripts. DAPI (blue) was used to label nuclei. (E, F) A portion of embryos injected with tbx2b MO and tbx2a cRNA exhibited a normal number of CS cells, but the average number of CS cells was significantly larger than the wild-type controls. A more robust rescue was seen in embryos injected with tbx2a MO and tbx2b cRNA, which on average had a similar number of CS cells compared to wild-type embryos. (G, H) DL lengths were rescued in some embryos by tbx2a cRNA in tbx2b morphants, and by tbx2b cRNA in tbx2a morphants, such that the average DL lengths were not significantly different from wild-type controls. (F, H) CS cells and DL lengths were counted in each nephron in 10 individuals in each treatment. (*p<0.05, **p<0.001, N.S.=not significant). Abbreviations: MO (morpholino), cRNA (capped RNA), DL (distal late), CS (corpuscle of Stannius). EXPRESSION / LABELING:
PHENOTYPE:
|
|
tbx2a/b are downstream of retinoic acid in the nephrogenesis pathway. Wild-type embryos were treated with exogenous RA at 3 dosages from 90% epiboly to the 28 ss. Wild-type embryos were also subjected to an RA inhibitor (DEAB) at 1.6×10-7 M at either 50% epiboly or 90% epiboly until fixation at 24 hpf. Whole mount in situ hybridization was then performed using riboprobes for tbx2a and tbx2b. Both the tbx2a and tbx2b domains decreased with at all three doses of RA, with total abrogation of expression occurring at 1×10-6 M. In contrast, DEAB treatment induced an expansion in the tbx2a/b domains at both treatment time points. This suggests that RA negatively regulates tbx2a/b expression, either directly or indirectly, in the development of the distal pronephros. At least 20 representative embryos were examined in each treatment. |
|
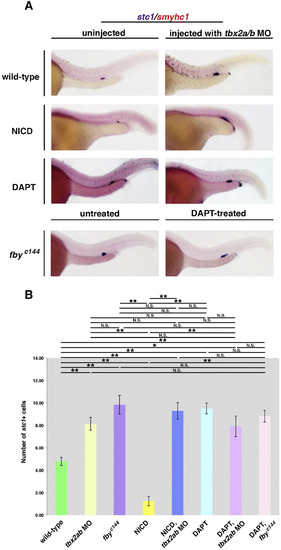
Notch signaling also inhibits CS differentiation, but in an upstream or independent way than tbx2a/b. NICD embryos were heat-shocked at 15 ss to ectopically activate Notch signaling and fixed at 24 hpf. Post heat-shock induction, NICD embryos exhibited a dramatic reduction of the CS, while siblings showed normal CS clusters. To block Notch signaling, wild-type embryos were incubated in 100 µm DAPT/E3 solution from 90% epiboly to the 24 hpf stage. DAPT treatment resulted in a significant elevation in the number of CS cells. Heat-shocked NICD tbx2a/b morphants exhibited a significant expansion in the CS that was in contrast to the reduced CS observed in uninjected heat-shocked NICD siblings. The number of cells in the these CS clusters was not significantly different to the expansion seen in wild-type embryos injected with tbx2a/b MO. DAPT-treated tbx2a/b morphants and fbyc144 mutants exhibited an expansion in the CS that was not significantly different than wild-type embryos treated with DAPT alone. Together, these results suggest that tbx2a/b are acting in the same pathway, downstream of Notch to suppress the CS. Representative images were based on thorough examination of at least 15 embryos per treatment. (*p<0.05, **p<0.001, N.S.=not significant).. |
|
tbx2a/b are expressed early in development and remain in the distal region of the developing pronephros. (A) Whole mount in situ hybridization using riboprobes for tbx2a and tbx2b reveal that these genes are expressed in the precursor tissue of the pronephros, the intermediate mesoderm, as early as the 8 ss. Both tbx2a and tbx2b continue to be expressed in the developing distal region of the pronephros through the 28 ss (24 hpf). (B) Sense probes for tbx2a and tbx2b applied to 28 ss wild-type embryos demonstrate that tbx2a/b expression in the pronephros is specific, rather than an artifact of the staining technique. |
|
tbx2a/b splice MO efficiency verification using RT-PCR. (A) Whole mount in situ hybridization reveals that tbx2a knockdown by tbx2a splice-blocking MO causes a decrease in the DL segment, marked by slc12a3. The tbx2a splice MO targets the splice acceptor site of intron 1, causing a fusion of exon 1 and exon 3. This change can be resolved by gel electrophoresis of RT-PCR wild-type and tbx2a splice morphant products. Sequencing revealed that the lower band present in morphants (asterisk) is the excised exon 2. (B) Similarly to effects of the tbx2a splice-blocking MO, the tbx2b splice-blocking MO causes a decrease in the DL segment. The tbx2b splice MO was designed to block the exon 3 splice donor site, resulting in an excision of exon 3. Sequencing revealed that the lower band present only in tbx2b splice morphant RT-PCR product is exon 3 from the tbx2b gene. |
|
tbx2a/b morpholino-knock down did not inhibit mesoderm formation. Embryos injected with tbx2ab MO at the 1-cell stage and fixed at the 3 ss. Whole mount in situ hybridization was then performed with a multitude of mesodermal markers. When compared to wild-type controls, the domains of these markers in morphants were not noticeably different, therefore suggesting that tbx2a/b is specifically active in pronephros segment cell fate choiceg. |
|
CS cell counting technique. (A) Wild-type 28 ss embryos were used to exemplify how CS cells were counted. Whole mount in situ hybridization was performed using stc1 or sim1a probes to label the CS cells. Embryos were then mounted dorsally in glycerol. On average, each nephron is composed of 4–6 cells per nephron. A black arrowhead indicates the location of each CS cell. (Top) The embryo has 2 cells in the first nephron, and 4 cells in the second nephron. (Bottom) Embryo two has 4 cells in the first nephron and three cells in the second nephron. |
|
tbx2a /boverexpression did not induce changes in the PCT, PST or DE. (A) While overexpression of tbx2a in wild-type embryos resulted in an increase in the DL, this overexpression did not result in any increases, decreases, or shifts in the PCT, PST, or DE domains. Likewise, tbx2b overexpression, while effective in altering the DL segment, was not able to initiate change in the PCT, PST, or DE domains. (B) Measuring the PCT, PST and DE in five representative embryos showed that these segments were not significantly different in length in embryos overexpressing tbx2a and tbx2b. (*p<0.05, **p<0.001, N.S. = not significant). |
Reprinted from Developmental Biology, 421(1), Drummond, B.E., Li, Y., Marra, A.N., Cheng, C.N., Wingert, R.A., The tbx2a/b transcription factors direct pronephros segmentation and corpuscle of Stannius formation in zebrafish, 52-66, Copyright (2017) with permission from Elsevier. Full text @ Dev. Biol.