- Title
-
miR-19b Regulates Ventricular Action Potential Duration in Zebrafish
- Authors
- Benz, A., Kossack, M., Auth, D., Seyler, C., Zitron, E., Juergensen, L., Katus, H.A., Hassel, D.
- Source
- Full text @ Sci. Rep.
|
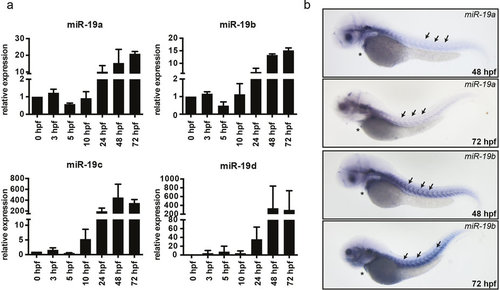
Expression of miR-19 in the zebrafish. (a) qRT-PCR analysis revealed that miR-19a-d expression is already detectable at very early stages of embryonic development and gets induced at 24 hpf. (±sd; n = 3 from 15 pooled embryos per sample) (b) In situ hybridization of miR-19a and miR-19b in 48 hpf and 72 hpf zebrafish embryos shows similar expression patterns in the heart (stars) and in skeletal muscle myosepts (arrows). EXPRESSION / LABELING:
|
|
Loss of miR-19 leads to bradycardia. (a) Lateral brightfield images of control-injected and MO19-injected 48 hpf zebrafish embryos. Note the cardiac edema and the blood congestion at the inflow tract developed in MO19 injected embryos. (b) Quantification of heart rate as heart beats per minute and ventricular fractional shortening as a measure for ventricular contractility in control (wt) and MO19 injected embryos. Morpholino-mediated knockdown of miR-19 in zebrafish leads to bradycardia and impaired ventricular contractility (±sd; n ≥ 14; p < 0.005). (c) qRT-PCR confirmed efficient knockdown of miR-19a-d. Notice the consistent reduction in miR-363 expression (±sd; n = 45 animals from 3 independent experiments). (d) Specific knockdown of miR-19a, miR-19c and miR-19d altered expression of neighboring miRNAs. However, knockdown of miR-19b did not interfere with expression of neighboring miRNAs (±sd; n = 45 animals from 3 independent experiments; p < 0.05). (e) 48 hpf zebrafish embryos injected with control- and MO19b-morpholino revealed that miR-19b deficiency is sufficient to mimic MO19-induced phenotype with characteristics of heart dysfunction. (f) miR-19b deficient embryos developed bradycardia with up to 36% ± 5.7% reduced heart frequency (± = sd; n ≥ 12; p < 0.005) (g,h) Relative changes in heart rate normalized to controls (dotted line) indicates that CRSIPR/Cas9 mediated knockout of miR-19b caused an identical and long-term reduction in heart rate as observed for MO19b (±sd; n = 10; p < 0.005). |
|
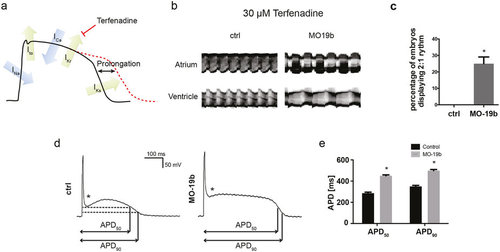
Loss of miR-19b induces prolongation of the action potential (a) Schematic illustration of an action potential with major depolarizing and repolarizing currents depicted. Terfenadine inhibits IKr current, thereby prolonging the ventricular action potential. (b) Atrial and ventricular m-mode recorded from control and MO19b-injected Tg(Myl7:GFP) zebrafish embryos after 30 μM Terfenadine treatment. (b,c) 25% ± 4.1% of miR-19b deficient embryos develop an AV-Block with the ventricle skipping every other contraction, whereas control injected embryos show no phenotype. (±sd; n = 3 with ≥10 animals per group; p < 0.05) (d,e) Patch-clamp Experiments revealed that morpholino-mediated loss of miR-19b results in prolonged action potentials with up to 57% ± 3.8% and 39% ± 3.5% prolongation of APD50 and APD90, respectively (±sd; n ≥ 10 from 5 hearts; p < 0.05). Additionally, the notch appeared to be less pronounced (stars). |
|
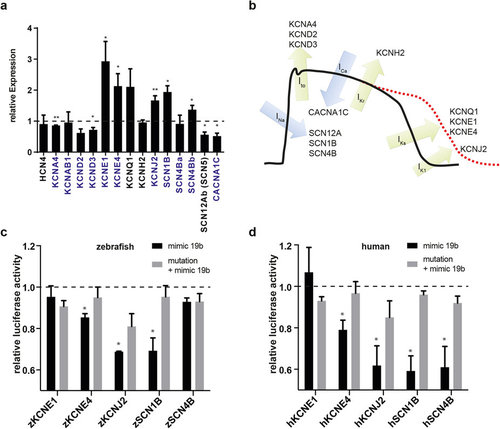
Loss of miR-19b results in dysregulation of cardiac ion currents. (a) qRT-PCR revealed dysregulation of various cardiac ion channels in miR-19b-deficient zebrafish. Transcripts with a predicted miR-19b binding site in its 3′-UTR are indicated in blue (±sd; n = 3 from 15 pooled embryos per sample; p < 0,05). (b) Illustration of a mammalian action potential with corresponding currents and genes. (c,d) Potential miR-19b targets were tested for direct regulation by a dual luciferase reporter gene assay. 3′UTR of indicated zebrafish (c) and human (d) genes was cloned downstream of a luciferase gene. Luciferase expression was normalized to renilla expression. As control, target sites were mutated to reverse miR-19b-induced repression. KCNE4, KCNJ2 and SCN1B proved to be direct targets in zebrafish and in humans. Additionally, the 3′UTR of human SCN4B showed responsiveness to miR-19b (±sd; n = 3; p < 0.05). |
|
Phenotype of reggae mutants is partially rescued by loss of miR-19b. (a) Quantification of heart rate in control injected, MO19b and co-injection of MO19b and MOkcne4 (n > 50 animals from 3 independent experiments; p < 0.005 using one-way ANOVA and unpaired t-test with Welch’s correction). (b) Schematic illustration of miR-19b function. (c) Heterozygous reggae mutants were rescued by miR-19b knockdown (±sd; n = 73 animals from 3 independent experiments; p < 0.05). PHENOTYPE:
|
|
Reduction of miR-363 (A) The morpholino targeting miR-363 (MO363 - green) was designed to bind the mature sequence of miR-363. (B) The Expression of miR-363 was reduced by 99 % by MO363-deficiency in zebrafish at 48 hpf (± sd; n=45 animals from 3 independent experiments; p<0,005). (C) miR-363 deficiency resulted in impaired eye development with reduced eye size up to complete loss of eyes. (D,E) Loss of miR-363 did not alter heart rate or contractility (± sd; n≥11). |
|
Heart Morphology after MO19b Application The overall morphology of the hearts in Tg(myl7:EGFP) zebrafish appeared to be not affected by reduction of miR-19b. v=ventricle; a=atrium. |
|
miR-19b-deficient zebrafish do not display defects in angiogenesis. Lateral brightfield (upper) and fluorescent (lower) images of control (left column) and MO-19 injected (right column) Tg(kdrl:GFP) transgenic zebrafish labeling all vascular endothelial cells in green at indicated timepoints. The fish display typical characteristics of heart dysfunction with blood congestion at the inflow tract and cardiac edema. However, the development of intersegmental vessels (arrowheads) and sub intestinal vessels (stars) was not noticeably impaired during the first five days of development. |
|
MOkcne4 testing and validation. (A) Lateral view of control- and MOkcne4 injected zebrafish embryos at 48 hpf. Note the pericardial edema and blood congestion at the inflow tract. (B, C) Quantification of heart rate (B) in control- and MOkcne4-injected embryos at 48 hpf documents a significant reduction in heart rate by KCNE4 knockdown without affecting contractility (C). (D) Luciferase reporter assay with the MOkcne4 recognizing translational start sequence from zebrafish kcne4 mRNA 5'- and in frame to the Luciferase open reading frame of the luciferase gene (pGL3kcne4). Coinjection of this construct with MOkcne4 (pGL3kcne4+MOkcne4) significantly repressed luciferase activity. Luciferase expression was normalized to renilla expression. (± sd; ***, p<0.005; three independent experiments; unpaired t-test with Welch’s correction). |