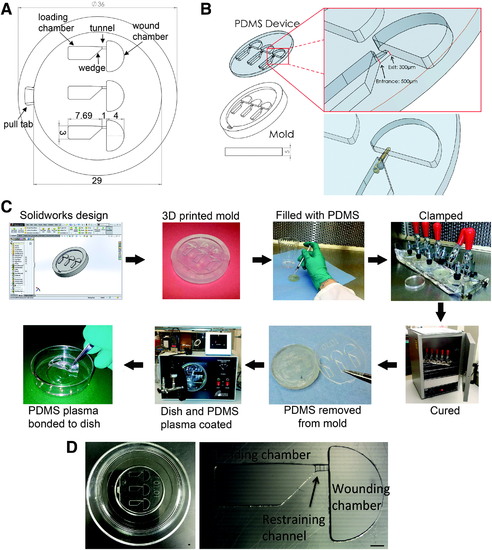
- Title
-
zWEDGI: Wounding and Entrapment Device for Imaging Live Zebrafish Larvae
- Authors
- Huemer, K., Squirrell, J.M., Swader, R., LeBert, D.C., Huttenlocher, A., Eliceiri, K.W.
- Source
- Full text @ Zebrafish
|
|
|
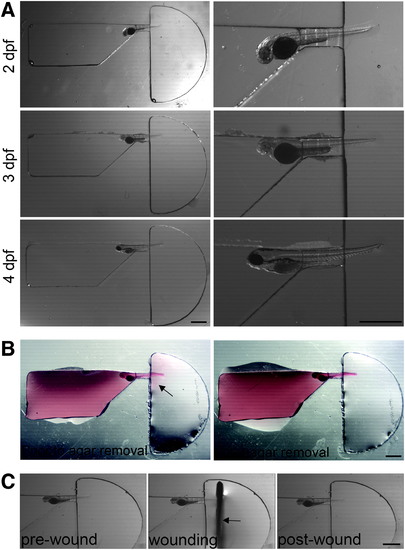
Positioning of larva in channel. (A) Dissecting microscope images showing 2, 3, and 4 dpf fixed larvae loaded into zWEDGI channel, with enlargement to show detail. (B) A minimal amount of agarose, labeled with rhodamine 6G and filling the loading chamber, exits from restraining tunnel into the wounding chamber, as detected by the pink halo (arrow). Residual agarose can be removed using a syringe needle with minimal impact on the tail, leaving the tail unrestrained in buffer in the wounding chamber. (C) Semicircular wounding chamber is designed to accommodate scalpel blade (arrow) for caudal fin transection. Scale bar = 1 mm. dpf, days postfertilization. |
|
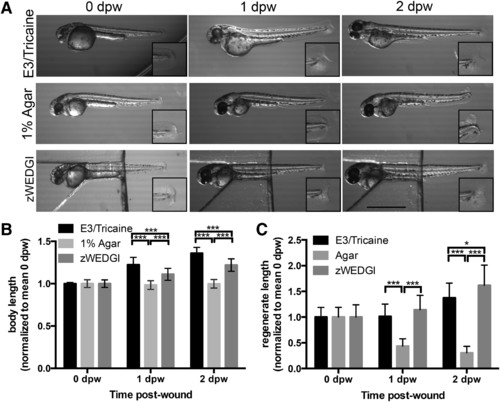
Growth and caudal fin regrowth are maintained in the zWEDGI. (A) Images from a dissecting microscope showing larvae collected within 30 min of wounding (0 dpw), 1 and 2 dpw that were anesthetized and maintained in E3 buffer (unrestrained), 1% agarose or zWEDGI with 1% agarose over head region and tail unrestrained in buffer. Scale bar = 1 mm. (B) Graph showing body length (eye to edge of tail fin) in the three mounting conditions. (C) Graph comparing regrowth (regenerate length) of transected tail fin with the three mounting conditions. Data normalized to mean of 0 dpw for a given treatment, n ≥ 28 larvae per treatment over a total of three replicates. **p < 0.01, ***p < 0.0001. dpw, days post-wound. |
|
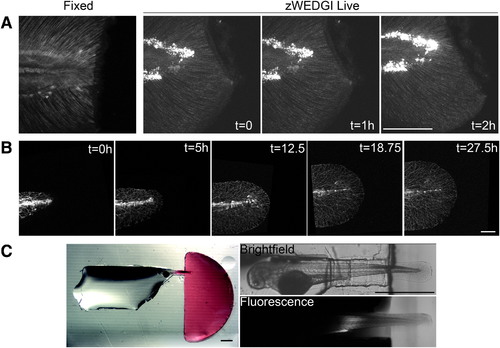
High-resolution light microscopy of larval tails in zWEDGI. (A) Z-projections of SHG images collected with similar imaging parameters of a fixed sample (single time point) and a live sample mounted in a zWEDGI (multiple time points), demonstrating short time frame imaging immediately after wounding. (B) Confocal long-term time lapse of tail development showing gfp-tagged neuron growth. (C) Dissecting microscope image with accompanying multiphoton image showing isolation of rhodamine 6G (red) to the wounding chamber, 2 h after initial application of the dye to the wounding chamber, illustrating only minor infiltration of dye into the restraining tunnel and levels below detection in the head region. (A, B) Scale bar = 100 μm. (C) Scale bar = 1 mm. SHG, second harmonic generation. |