- Title
-
Sox9b is a mediator of retinoic acid signaling restricting endocrine progenitor differentiation
- Authors
- Huang, W., Beer, R.L., Delaspre, F., Wang, G., Edelman, H.E., Park, H., Azuma, M., Parsons, M.J.
- Source
- Full text @ Dev. Biol.
|
RA and Notch signaling both influence larval CAC expression of sox9b. (A) Model of the regulation controlling larval CAC differentiation towards an endocrine fate. Notch signaling blocks differentiation that can be overcome by the Notch-inhibitor, DAPT. RA also impedes differentiation, an action that can be inhibited either by blocking RA synthesis with diethylaminobenzaldehyde (DEAB) or signal transduction with a dominant negative RA receptor (dnRAR). DAPT and DEAB work synergistically to inhibit CAC differentiation, suggesting a shared mechanism downstream of Notch and RA signaling ( Huang et al., 2014). (B-F) Images of dissected larval foregut following whole mount in situ hybridization to detect sox9b expression. Larvae were treated from 3 to 5 dpf with DMSO (B), RA (C), DEAB (D), DAPT (E) or RA+DAPT (F). White dashed lines outline pancreata. Scale bar=100 µm (F). (G) Quantitative RT-PCR results indicate that sox9b expression is significantly increased in dissected larval pancreata following RA treatment from 4 to 5 dpf; t-test *p<0.01. (H) Quantification of the number of Nkx6.1+ CACs in larval pancreata at 5 dpf after drug treatment from 3 to 5 dpf. Error bars indicate SD. |
|
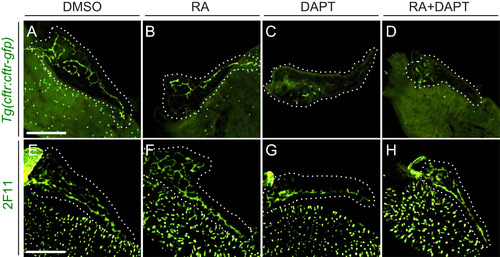
RA and Notch signaling have separate effects on CAC differentiation. (A-D) Images of 5 dpf cftr:cftr-gfp dissected foregut following drug treatment. GFP signal is abolished in larvae treated with DAPT (C) and RA+DAPT (D). (E-H) Images of anti-2F11 staining in 5 dpf dissected foregut following drug treatment. Expression of the ductal epithelial marker 2F11 is maintained under all treatment conditions. Larvae were incubated from 3 to 5 dpf with DMSO (A, E), RA (B, F), DAPT (C, G), or RA+DAPT (D, H). White dashed lines outline pancreata. Scale bar=100 µm. |
|
Inducible systems to overexpress Sox9b or dominant-negative Sox9b. (A) sox9b and (F) trsox9b cre responder constructs used to make new lines. Tol2 arms (gray triangles) facilitate transgenesis. Following cre-recombination between LoxP sites (black triangles), both the eCFP gene (blue) and stop sequences (yellow) are removed allowing ubiquitous expression of either sox9b-2A-mCherry (A) or trsox9b-2A-mCherry (F) to be driven off the ubb promoter/enhancer. Due to 2A sequence (green), either Sox9b (A) or trSox9b (F) will be expressed as separate proteins along with mCherry. Double transgenic larvae for the ubiquitous creERT2driver and either sox9b cre-responder (B-E) or trsox9b cre-responder (G-J). (B, D, G, J) Merged fluorescence images of the larvae at 72 hpf to detect both eCFP in blue and mCherry in red. (C, E, I, L) Results of whole mount in situ hybridization on 24 hpf larvae to detect the known Sox9 target gene col2a1a. (H, K) Bright field dorsal images at 72 hpf. From 4 hpf, fish in (B, C, G-I) were treated with vehicle and fish in (D, E, J-L) were treated with 5 ¼M 4OHT. 4OHT treatment leads to induction of mCherry expression (D and J) concomitant with abnormal gross development due to Sox9b overexpression (D) and trSox9b expression (J-K). Overexpression of Sox9b also caused increased expression of the Sox9 target gene col2a1a (E). Scale bars=500 µm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Inducible expression of Sox9b and truncated Sox9b in CAC pancreatic progenitors. (A, B and D, E) Confocal images of 5 dpf pancreata dissected from double transgenic larvae for the Notch-responsive creERT2driver and either sox9b cre-responder (A, B) or trsox9b cre-responder (D, E) showing localization of Nkx6.1 (green) and mCherry (red). Transgenic larvae were treated with vehicle (A, D) or 4OHT (B, E) from 2 to 3 dpf. The numbers of double positive (green/red) cells were quantified (C, F). Overexpression of Sox9b did not significantly affect numbers of Nkx6.1 positive CACs (C); however, 4OHT induction of trSox9b significantly reduced CAC numbers, an effect that was heightened if 4OHT treatment was performed even earlier in development (1.5-2.5 dpf, F). Scale Bar=100 µm, *p<0.05, **p<0.001. |
|
RA regulates CAC differentiation through action of Sox9b. (A) Experimental timeline of 4OHT-dependent induction of either Sox9b or truncated-Sox9b expression. 4OHT was applied from 2 to 3 dpf, and drugs (either DMSO (vehicle), RA, DAPT or RA+DAPT) from 3 to 5 dpf. (B-I, and K-R) Confocal images of 5 dpf dissected pancreata from larvae transgenic for the Notch-responsive creERT2driver, a pan-endocrine marker neuroD:GFP and either the sox9b cre responder (B-I) or the truncated sox9b cre responder (K-R). All larvae were incubated from 2 to 3 dpf with either vehicle or 4OHT (as indicated). Next, vehicle and 4OHT treated larvae were incubated from 3 to 5 dpf with either: DMSO (B, C, K, L), RA (D, E, M, N), DAPT (F, G, O, P), or RA+DAPT (H, I, Q, R). 2° islet cellsn are marked by GFP (white arrow heads) and seen in the tail of the pancreas. White dashes outline the pancreata. Scale bar=100 µm. Average number of 2° islet cells/pancreas in larvae transgenic for the Notch-responsive creERT2driver and Tg(neuroD:GFP)nl1 and either the sox9b cre responder (J) or the truncated sox9b cre responder (S). N=number of larval pancreata quantified. Error bar represents SE; *p<0.01, **p<0.001. EXPRESSION / LABELING:
PHENOTYPE:
|
|
sox9b haploinsufficiency impedes the effects of RA on CAC differentiation. (A) Immunofluorescence staining using a Sox9b antibody in 5 dpf pancreata expressing Tp1:nuc-mCherry. Sox9b expression co-localizes with nuclear mCherry in CACs (white arrows). Pancreas is outlined in dashed line. Scale bar=20 µm. (B) Western blot demonstrating that Sox9b expression is reduced in sox9bfh313 heterozygous larvae. (C-F) Images of dissected pancreata from 5 dpf larvae with the following genotype: (C-D) wildtype (sox9b+/+) (E-F) sox9bfh313 heterozygous (sox9b+/). in situ hybridization for neuroD was used to detect 2° islets. Larvae of differing genotypes (as indicated) were treated from 3 to 5 dpf with either DAPT (C, E) alone or RA+DAPT (D, F). 2° islets are indicated by black arrow heads. White dashes outline the pancreata. Scale bar=100 µm. (G) Numbers of CACs quantified using expression of Tp1:eGFP in pancreata from 5 dpf larvae, genotyped as indicated. Heterozygotes do not have significantly different numbers of CACs compared to wildtype. (H) Numbers of 2° islets/pancreas from genotyped larvae after being treated with DAPT alone or RA+DAPT. Error bar represents SD; **p<0.001. |
|
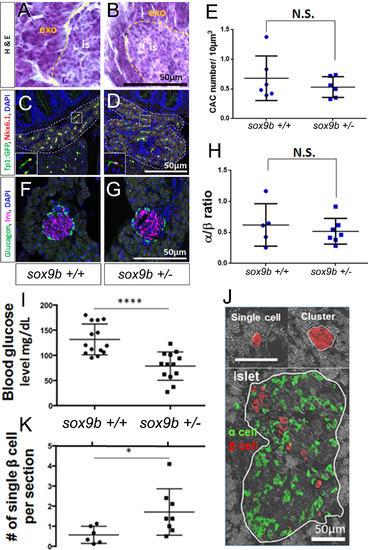
β-cell regeneration is accelerated in adult sox9b heterozygous fish. Hematoxylin and eosin stained sections of adult pancreata from: A) wildtype (here on sox9b+/+); and, B sox9bfh313 heterozygotes (here on sox9b+/-). Exocrine (exo) and islet (is). (C, D) Detection of CACs using immunofluorescent detection of Nkx6.1 and GFP on pancreas sections from fish transgenic for the Notch-responsive reporter and either sox9b+/+ (C) or sox9b+/- (D). Double positive (Nkx6.1+/GFP+) cells were quantified and show no significant difference between sox9b+/+ and sox9b+/- (E). (F, G) Immunofluorescent detection of glucagon (green) and insulin (magenta) to label α and β cells in sox9b+/+ (F) and sox9b+/- (G) adult pancreata. (H) α to β ratio showed no significance difference between islet composition from sox9b+/+ and sox9b+/- fish. (I-K) After β-cell ablation, sox9b+/- adult fish showed better capability of β-cell regeneration compared to sox9b+/+. (I) Blood glucose at 7 days post β-cell ablation shows significantly lower blood glucose levels in sox9b+/- (N=13) compared to sox9b+/+ (N=14). (J) Representative immunofluorescent images of single β cells, small β-cell clusters and pre-existing islets. (K) Number of single β cells 7 days post β-cell ablation was significantly higher in sox9b+/- (n=8) than that in sox9b+/+ (n=6). Each dot represents the average number of single β cells observed per section from one fish. 18-20 transverse paraffin sections for each fish were examined. In this manner we analyzed the majority of pancreatic tissue for all 4 lobes. *p<0.05, ****p<0.0001. |
Reprinted from Developmental Biology, 418(1), Huang, W., Beer, R.L., Delaspre, F., Wang, G., Edelman, H.E., Park, H., Azuma, M., Parsons, M.J., Sox9b is a mediator of retinoic acid signaling restricting endocrine progenitor differentiation, 28-39, Copyright (2016) with permission from Elsevier. Full text @ Dev. Biol.