- Title
-
Enhanced angiogenesis, hypoxia and neutrophil recruitment during Myc-induced liver tumorigenesis in zebrafish
- Authors
- Zhao, Y., Huang, X., Ding, T.W., Gong, Z.
- Source
- Full text @ Sci. Rep.
|
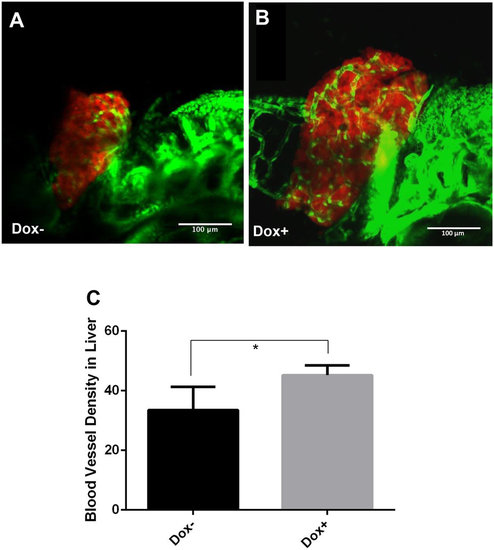
Enhanced angiogenesis in the liver by induction of transgenic Myc expression in TO(Myc) larvae. Tg(Fli1:EGFP), LiPan and TO(Myc) zebrafish were crossed to generate triple transgenic zebrafish larvae. The liver was labeled by DsRed expression and blood vessel by EGFP expression. Triple transgenic larvae were treated with 30 µg/ml Dox from 3 dpf to 7 dpf and imaged by a confocal microscope. (A) A representative triple transgenic larva without Dox treatment. (B) A representative triple transgenic larva with Dox treatment. (C) Blood vessel density. Blood vessel density is defined by the ratio of blood vessel area (green) over the total liver area (as demarcated by DsRed expression) and measured by online ImageJ software. The quantitative data were based on 10 samples per group. The original magnification was 40x. Standard error bars are indicated and the two groups show significant difference with P-value < 0.05 by unpaired t-test statistical analysis. Scale bars = 100 µm. |
|
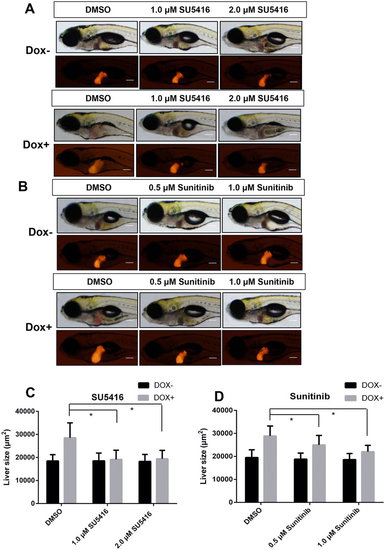
Effects of angiogenesis inhibitors on tumorigenic liver growth. To(Myc)/LiPan double transgenic larvae were treated with anti-angiogenesis compounds SU5416 (1 µM or 2 µM) or sunitinib 0.5 µM or 1 µM) with or without 30 µg/ml Dox from 3 dpf to 7 dpf. 0.1% DMSO was used as vehicle control for both compounds. Liver areas were imaged and 2D liver areas were quantified. (A,B) Images of representative 7-dpf double transgenic larvae treated with different concentrations of SU5416 (A) and sunitinib (B). Both bright field (top) and fluorescent images (bottom) are shown. (C,D) Quantification of changes of 2D liver size after treatment with SU5416 (C) and sunitinib (D). The quantitative data were based on 10 samples per concentration group. The original magnification was 10x. Data are represented as mean ± SD. Astrisks indicate significant difference with P-value < 0.05 by two way ANOVA statistical analysis. Scale bars = 100 µm. |
|
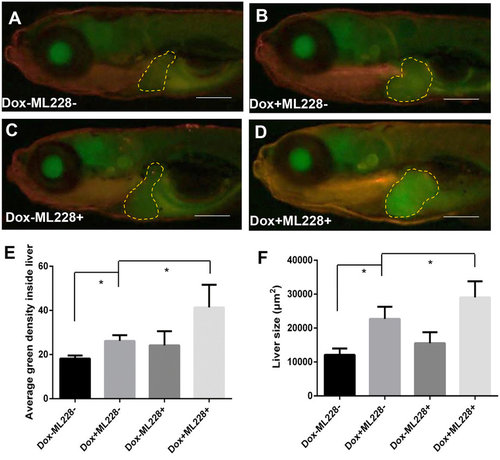
Stimulation of tumorigenic liver growth by hypoxia. TO(Myc) and Tg(phd3::EGFP) double transgenic larvae were generated and induced by Dox for 4 days from 4 dpf to 7 dpf. (A-D) Images of liver hypoxia as indicated by EGFP expression in dashline-circled liver areas. A non-Dox treated control is shown in (A) and a Dox treated larva is in (B). 0.5 µM ML228 was used to enhance hypoxia and representative images are shown in (C,D) in the absence or presence of Dox, respectively. The original magnification was 20x. (E) Quantification of level of hypoxia as indicated by average GFP green density inside the liver. (F) Quantification of 2D liver size. The quantitative data were based on 5 samples per concentration group. Data are represented as mean ± SD. Astrisks indicate significant difference with P-value < 0.05 by unpaired t-test statistical analysis. Scale bars = 100 µm. |
|
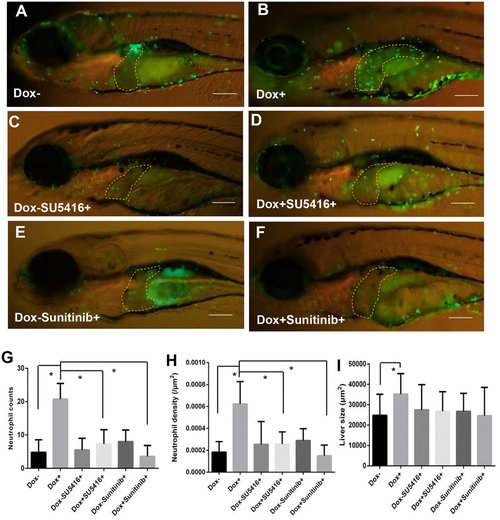
Enhanced tumor infiltration of neutrophils by induced Myc expression and suppression of neutrophil infiltration by angiogenesis inhibitors. TO(Myc) and Tg(mpx:EGFP) double transgenic larvae were generated with EGFP labeled neutrophils. (A-F) Images of the double transgenic larvae in the presence of the following chemicals: nil control (A), Dox (B), SU5416 (C), Dox+SU5416 (D), sunitinib (E) and Dox+sunitinib (F). The larvae were treated from 4 dpf to 7 dpf. In all images, the liver areas are circled by dashlines. The original magnification was 20x. (G) Neutrophil counts in the liver. (H) Neutrophil density in the liver. Neutrophil density was calculated as number of neutrophils per µm2. (I) Quantification of 2D liver size. The quantitative data were based on 10 samples per concentration group. Data are represented as mean ± SD. Astrisks indicate significant difference with P-value < 0.05 by unpaired t-test statistical analysis. |
|
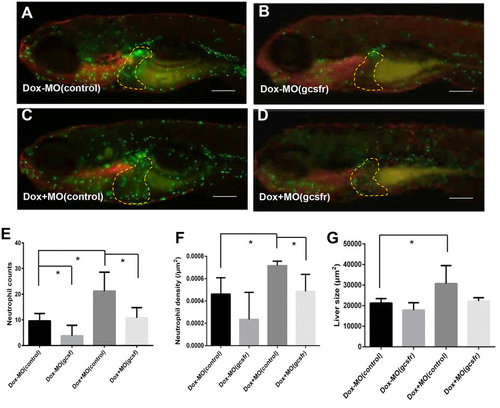
Stimulation of tumorigenic liver growth by tumor-infiltrated neutrophils. TO(Myc) and Tg(mpx:EGFP) double transgenic larvae were used for norpholino knockdown of Gcsfr to inhibit neutrophil differentiation. Morpholino oligonucleotides were injected into the embryos at 1-2-cell stage. (A-D) Images of the double transgenic larvae after injection of either MO(control) (A,C) or MO(gcsfr) (B,D). These injected embryos were either treated with Dox (C,D) or without Dox from 4 dpf to 7 dpf (A,B). The liver areas are circled. The original magnification was 20x. (E) Neutrophil counts in the liver. (F) Neutrophil density in the liver under different conditions. (G) Quantification of 2D liver size. The quantitative data were based on 5 samples per concentration group. Data are represented as mean ± SD. Asterisks indicate significant difference with P-value < 0.05 by unpaired t-test statistical analysis. Scale bars = 100 µm. |
|
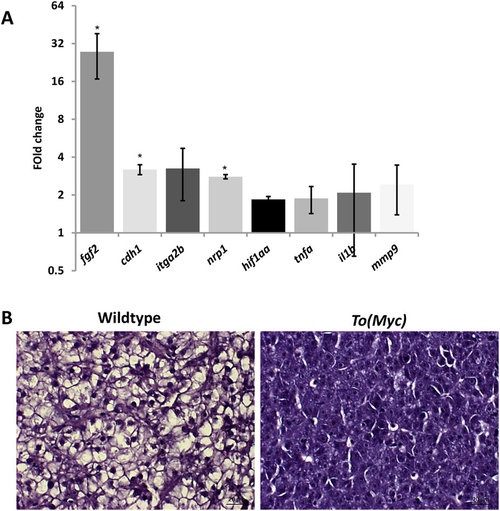
Molecular and histological characterization of Myc overexpressed livers. One-month-old wildtype or TO(Myc) zebrafish were treated with 30 µg/ml Dox for 7 days and euthanized for RNA extraction and histological analyses. (A) Validation of increased angiogenesis, hypoxia and inflammatory response by biomarker gene expression. RNA expression of selected biomarker genes were measure by RT-qPCR. Fold changes shown are ratio of the values from To(Myc) fish over wildtype fish after calibration with beta-actin mRNA as an internal control. Asterisks indicate significant difference with P-value < 0.05 by t-test among the three biological replicates. (B) Histological comparison of livers from wildtype (left) and TO(Myc) (right) fish. Fish were treated with or without Dox (30 µg/ml) from 4 dpf to 7 dpf. Representative pictures are shown for each group (n = 10 per group). The original magnification was 100x. Scale bars = 20 µm. EXPRESSION / LABELING:
PHENOTYPE:
|