- Title
-
Mycobacterial Acid Tolerance Enables Phagolysosomal Survival and Establishment of Tuberculous Infection In Vivo
- Authors
- Levitte, S., Adams, K.N., Berg, R.D., Cosma, C.L., Urdahl, K.B., Ramakrishnan, L.
- Source
- Full text @ Cell Host Microbe
|
In Vivo Phagolysosomal Trafficking of M. marinum (A) Illustration of zebrafish larva with injection sites outlined in red. The hindbrain ventricle (HBV) is accessible to recruited myeloid cells, while the caudal vein (CV) traverses the caudal hematopoietic tissue where myeloid cells develop. (B) Confocal images of blue fluorescent Mm that have been phagocytosed by green fluorescent macrophages in the brain of a 2 day post-fertilization (dpf) larva stained with red LT; one bacterium shown colocalizes with LT, and the other does not. Scale bar, 10 µm. (C) Percent of Mm colocalizing with LT dye 24 hr post infection (hpf) into the HBV, representative of three experiments. (D) Percent of Mm colocalizing with LT dye at 3, 8, and 24 hpi in the CV, representative of three experiments. (E) Confocal images of blue fluorescent Mm that were pre-labeled with red pHrodo prior to infection into the HBV of 2dpf larvae with green fluorescent macrophages; bacteria that colocalize with pHrodo (arrowheads) and one that does not (arrow). Scale bar, 10 µm. (F) Percent of Mm colocalizing with pHrodo at 3 and 24 hpi in the HBV or CV of a 3 dpf larva, representative of two experiments. (G and H) Percent of Mm colocalizing with DQ-BSA (G) or MR-Cathepsin (MRC) (H) imaged at 24 hpi following infection in the HBV or CV of 2 dpf larvae, representative of two experiments each. (I) Percent of live, heat-killed, or ptpA::Tn Mm pre-labeled with pHrodo prior to infection into the HBV of 2 dpf larvae imaged at 24 hpi, representative of three experiments. (J) Percent of ΔESX-1 Mm colocalizing with pHrodo imaged at 24 hpi in the HBV of 2 dpf larvae, representative of three experiments. Significance tested using one-way ANOVA with Tukey′s post-test (F and I) or two-tailed unpaired t test (G, H, and J). Each point in (C), (D), and (F)-(J) represents one larva, with mean depicted as a horizontal line. See also Figures S1 and S2. |
|
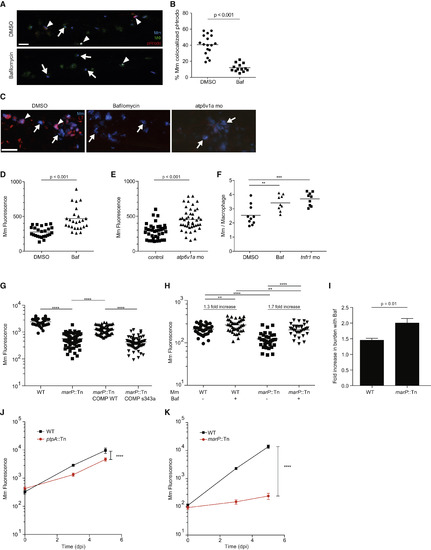
Lysosomal Trafficking Is a Host-Beneficial Process, which Is Counteracted by Bacterial MarP (A) Confocal images of 3 dpf larvae that were infected with Mm in the CV at 2 dpf and then treated for 24 hr with 50 nM Bafilomycin (Baf) or DMSO control. Arrowheads denote bacteria labeled with pHrodo, while arrows show pHrodo-negative bacteria. (B) Percent of Mm colocalizing with pHrodo at 24 hpi as shown in (A), representative of two experiments. (C) Confocal images of 3 dpf larvae that were infected in the CV with blue fluorescent Mm at 2 dpf and stained with red LT at 3 dpf following treatment with DMSO, 50 nM Baf, or morpholino targeting atp6v1a at 0 dpf. Arrowheads denote bacteria that colocalize with LT, while arrows denote LT-negative bacteria. (D) Bacterial burden (FPC) measured at 24 hpi with 300 Mm; fish were randomly assigned to DMSO or 50 nM Baf treatment immediately after infection, representative of three experiments. (E) Bacterial burden measured at 24 hpi with 300 Mm in larvae injected at 0 dpf with a morpholino targeting atp6v1a or control, representative of two experiments. (F) Average intramacrophage burden at 40 hpi with 60 Mm into the CV comparing larvae treated with DMSO or 50 nM Baf following infection, or injected with a morpholino targeting tnfr1 at 0 dpf, which was used as a positive control, representative of three experiments. (G) Bacterial burden measured at 3 dpi following infection with wild-type Mm, marP::Tn, marP::Tn transformed with a plasmid containing Mtb marP, or marP::Tn transformed with a plasmid containing Mtb marP with a mutation in the active site serine (S343A), representative of two experiments. (H) Bacterial burden at 1 dpi following infection with wild-type or marP::Tn Mm and treatment with either DMSO or 50 nM Baf, representative of three experiments. The fold increase in burden following treatment with Baf is shown for wild-type and marP::Tn. (I) Fold increase in bacterial burden during infection with wild-type or marP::Tn Mm following treatment with 50nM Baf. Shown is the average of four experiments as in (H) ± SEM. (J) Bacterial burden measured at 0, 3, and 5 dpi following infection with 250 wild-type or ptpA::Tn Mm with mean and 95% CI shown, representative of three experiments. (K) Bacterial burden at 0, 3, and 5 dpi following infection with wild-type or marP::Tn Mm with mean and 95% CI shown, representative of three experiments. Significance tested using two-tailed unpaired t test (B, D, E, and I-K) or one-way ANOVA with Tukey’s post-test (F-H). Each point in (B) and (D)-(H) represents one larva. See also Figure S3. |
|
Lysosomal Trafficking Fails to Eradicate Mm Infection (A) Cartoon diagram of infectivity experiment. Scale bar, 10 µm. (B) Percent of zebrafish larvae still infected at 60 hpi following infection in the HBV with single pHrodo-labeled Mm and sorting into acidified/non-acidified groups, representative of three experiments. (C) Photobleaching assay to discern live from killed bacteria at the end of the infectivity assay showing a live bacterium that recovers fluorescence and a dead one that does not. Scale bar, 5 µm. (D) Data in (B) amended so that larvae with only non-recovering bacteria are placed into the uninfected category, representative of three experiments. (E) Bacterial numbers at the end of the infectivity experiment, showing only bacteria that recovered after photobleaching, representative of three experiments. p values in parentheses reflect the statistical significance of comparing each larva in that group to the starting bacterial number (1 in each fish) in a Wilcoxon matched-pair signed rank test. (F-H) Percentage of zebrafish larvae still infected at 60 hpi which contained pHrodo-labeled marP::Tn (F), ptpA::Tn (G), and ΔESX-1 (H) Mm separated by whether the bacteria were pHrodo-positive (lysosomal) or pHrodo-negative (phagosomal) at sorting, representative of two (ΔESX-1) or three (marP::Tn, ptpA::Tn) experiments. Significance tested using two-tailed unpaired t test (E) or Fisher’s exact test (B, D, and F-H). Each point in (E) represents one larva. See also Figure S4. |